the reaction 2a → a2 was experimentally determined to be second order with a rate constant, k, equal to 0.0265 m–1min–1. if the initial concentration of a was 3.75 m, what was the concentration of a (in m) after 180.0 min?
Answers
Based on the integrated equation used for reactant concentration calculation in a second-order reaction and the data given (initial concentration, reaction rate constant, and time elapsed), the concentration of A after 180.0 min will be 0.20 M.
The integrated equation for the reactant concentration in a second-order reaction looks like this:
1/[A] = kt + 1/[A]₀
k - reaction rate constant (0.0265 min⁻¹M⁻¹)
[A]₀ - initial concentration (3.75 M)
t - time elapsed (180.0 min)
1/[A] = 0.0265 min⁻¹M⁻¹ * 180.0 min + 1/(3.75 M) = 4.77 M⁻¹ + 0.27 M⁻¹ = 5.04 M⁻¹
[A] = 1 / (5.04 M⁻¹) = 0.20 M
You can learn more about second-order reactions here:
brainly.com/question/12446045
#SPJ4
Related Questions
How many atoms are in 1.22 moles of magnesium?
Answers
Answer:
7.34684 × 10^23 atoms
Explanation:
Well, one mole is basically
6.022 × 10^23 atoms. So 1.22 moles would be
6.022 × 10^23 ⋅ 1.22 atoms.
6.022 × 10^23 ⋅ 1.22 = 7.34684 × 10^23 atoms
The total number of atoms in 1.22 moles of magnesium is 7.34 × 10²³.
One mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions).The number 6.022 × 10²³ is known as Avogadro's number or Avogadro's constant.Given:
1.22 moles of magnesium
\(1 \text{ mole}= 6.022*10^{23}\text{ atoms}\\\\1.22 \text{ moles}= 1.22*6.022*10^{23}\text{ atoms}\\\\1.22 \text{ moles}=7.34*10^{23}\text{ atoms}\)
So, the total number of atoms in 1.22 moles of magnesium is 7.34 × 10²³.
Learn more:
brainly.com/question/11175128
hydrogen- is radioactive and has a half life of years. calculate the activity of a sample of hydrogen- . give your answer in becquerels and in curies. round your answer to significant digit.
Answers
The activity of a sample of hydrogen- , rounded to the nearest significant digit, is N × 0.00693 Bq and N × 2.56 × 10⁻¹² Ci.
What is sample?Sample in chemistry is a small amount of a substance that is used to conduct a chemical analysis. It is often taken from a larger quantity of a material and used to determine the composition or properties of the material. For example, a chemist may take a sample of a compound and analyze it to determine its melting point and boiling point.
The activity A of a sample of a radioactive material is the number of radioactive decays per unit time. The half-life of a radioactive material is the time it takes for half of the original amount of material to decay.
For a sample of hydrogen- , the activity A can be calculated using the equation A = N × 0.693/t, where N is the initial number of atoms in the sample and t is the half-life of hydrogen- (in years).
Given that the half-life of hydrogen- is years, the activity A in becquerels (Bq) is:
A = N × 0.693/t = N × 0.693/ = N × 0.00693
The activity A in curies (Ci) can be calculated by multiplying the activity in becquerels by 3.7 × 10⁻¹⁰:
A = N × 0.00693 × 3.7 × 10-10
Therefore, the activity of a sample of hydrogen- , rounded to the nearest significant digit, is N × 0.00693 Bq and N × 2.56 × 10-12 Ci.
To learn more about sample
https://brainly.com/question/25736513
#SPJ4
Calculate the molecular mass or formula mass (in amu) of each of the following substances: (a) PCl5 amu (b) C4H10 amu (c) NF2 amu (d) Al2O3 amu (e) Fe(NO3)3 amu (f) Mg3N2 amu (g) (NH4)2CO3 amu
Answers
Molecular mass of PCl5 = (1 x atomic mass of P) + (5 x atomic mass of Cl)
Atomic mass of P = 30.97 u
Atomic mass of Cl = 35.45 u
Molecular mass of PCl5 = (1 x 30.97 u) + (5 x 35.45 u) = 208.23 u
(b) C4H10:
Molecular mass of C4H10 = (4 x atomic mass of C) + (10 x atomic mass of H)
Atomic mass of C = 12.01 u
Atomic mass of H = 1.01 u
Molecular mass of C4H10 = (4 x 12.01 u) + (10 x 1.01 u) = 58.12 u
(c) NF2:
Formula mass of NF2 = (1 x atomic mass of N) + (2 x atomic mass of F)
Atomic mass of N = 14.01 u
Atomic mass of F = 18.99 u
Formula mass of NF2 = (1 x 14.01 u) + (2 x 18.99 u) = 52.99 u
(d) Al2O3:
Formula mass of Al2O3 = (2 x atomic mass of Al) + (3 x atomic mass of O)
Atomic mass of Al = 26.98 u
Atomic mass of O = 16.00 u
Formula mass of Al2O3 = (2 x 26.98 u) + (3 x 16.00 u) = 101.96 u
(e) Fe(NO3)3:
Formula mass of Fe(NO3)3 = (1 x atomic mass of Fe) + (3 x (1 x atomic mass of N + 3 x atomic mass of O))
Atomic mass of Fe = 55.85 u
Atomic mass of N = 14.01 u
Atomic mass of O = 16.00 u
Formula mass of Fe(NO3)3 = (1 x 55.85 u) + (3 x (1 x 14.01 u + 3 x 16.00 u)) = 241.99 u
(f) Mg3N2:
Formula mass of Mg3N2 = (3 x atomic mass of Mg) + (2 x atomic mass of N)
Atomic mass of Mg = 24.
Write the structure of two isomers of hydrocarbon
Answers
Answer:
not sure
Explanation:
what type of cutting tool cuts primarily with the sides of the tool instead of the end or face?
Answers
The type of cutting tool cuts primarily with the sides of the tool instead of the end or face is trochoidal paths, the swarf milling, not the Side mill and not the Center drill.
The roughing cuts are these days most usually done with the trochoidal paths, which are the primarily the side cutting technique. when the cutting is for softer materials, the cutting tool should be set at the negative angle in order to be reduce the amount of the material that is removed.
For the swarf milling we should use the side of the tool to mill the vertical wall that is relative to the tool. On the five axis mill, we can create helical and the other developable surfaces in this way.
To learn more about cutting here
https://brainly.com/question/30532377
#SPJ4
in chemistry class, students have been discussing the differences between physical and chemical changes. the students carried out several procedures and recorded their observations. the students determined that one of the procedures is an example of a physical change, but not a chemical change.
Answers
Answer:
C, Reactants rearrange to form new products
Explanation:
2. The energy of a photon that has a wavelength of 12.3 nm is _______ J.
A) 2.72 x 10^-50
B) 1.62 x 10^-17
C) 4.42 x 10^-23
D) 1.99 x 10^-25
E) 1.51 x 10^-17
Answers
Why do you think the Sun is not at the center of Mercury’s orbit?
Answers
Answer:
Like Venus, Mercury orbits the Sun within Earth's orbit as an inferior planet, and its apparent distance from the Sun as viewed from Earth never exceeds 28°. This proximity to the Sun means the planet can only be seen near the western horizon after sunset or the eastern horizon before sunrise, usually in twilight.
Explanation:
Please help! Picture is provided.

Answers
Answer:
I honestly dont know Im not good at chem:( I love your pfp tho so cute<3 Have a nice day
Explanation:
Answer:
Bro I don't know pls bear I am so ungrateful I am a noob
If+you+have+a+10%+sugar+solution+and+a+35%+sugar+solution,+how+does+the+10%+solution+compare+to+the+35%+solution?
Answers
The percentage represents the proportion of sugar in the solution by weight. The 10% solution contains a lower amount of sugar compared to the 35% solution, indicating that it is more diluted and has a lesser sugar content.
The concentration of a solution is determined by the ratio of the amount of solute to the amount of solvent. In this case, the 10% sugar solution contains 10 grams of sugar dissolved in every 100 milliliters of solution. On the other hand, the 35% sugar solution contains 35 grams of sugar dissolved in every 100 milliliters of solution.
Comparing the two solutions, the 10% solution has a lower sugar content and is more diluted compared to the 35% solution. This means that the 10% solution has a higher proportion of water (solvent) in relation to sugar (solute) than the 35% solution. In terms of taste or sweetness, the 35% solution would be significantly sweeter due to its higher sugar concentration.
Learn more about dissolved here: brainly.com/question/2364287
#SPJ11
Nasogastric Intubation and Enteral Feedings: Priority Actions for an Adolescent (RN QSEN - Safety , Active Learning
Template - Nursing Skill, RM FUND RN 9.0 Ch 54)
prepare the formula, tubing, and infusion device.
Check expiration dates, and note the content of the formula.Ensure that the formula is at room temperature.
Set up the feeding system via gravity or pump.
Mix or shake the formula, fill the container, prime the tubing, and clamp it.Assist the client to Fowler's position, or elevate the head of the bed to a minimum of 30°.Auscultate for bowel sounds.monitor tube placement.
Check gastric contents for pH. A good indication of appropriate placement is obtaining gastric contents with a pH between 0 and 4.Aspirate for residual volume.
Flush the tubing with at least 30 mL tap water.Administer the formula
Answers
Nasogastric intubation is a medical procedure in which a flexible tube is inserted through the nose and passed down the throat into the stomach. Enteral feedings refer to the delivery of nutrition directly into the gastrointestinal tract, specifically the stomach or small intestine
To prioritize actions for nasogastric intubation and enteral feedings in an adolescent, the following steps can be taken:
1. Prepare the formula, tubing, and infusion device: Gather all the necessary equipment required for the procedure, including the enteral feeding formula, appropriate tubing, and the infusion device (if using a pump). Ensure that the equipment is clean and in proper working condition.
2. Check expiration dates and note the content of the formula: Verify the expiration dates of the enteral feeding formula and discard any expired products. Take note of the nutritional content and any specific instructions provided by the healthcare provider regarding the formula.
3. Ensure that the formula is at room temperature: Some enteral formulas may require warming to room temperature before administration. Follow the manufacturer's instructions for warming if necessary, ensuring the formula is not too hot to avoid causing discomfort or harm.
4. Set up the feeding system via gravity or pump: Depending on the healthcare provider's order, set up the feeding system using either gravity or a pump. Follow the manufacturer's instructions for assembling the system correctly and securely.
5. Mix or shake the formula, fill the container, prime the tubing, and clamp it: If using powdered formula, mix it with the appropriate amount of water as directed by the manufacturer. Shake the formula well to ensure it is adequately mixed. Fill the container with the prepared formula and connect it to the tubing. Prime the tubing by allowing the formula to flow through it until all the air bubbles are removed. Clamp the tubing to prevent any spillage.
6. Assist the client to Fowler's position or elevate the head of the bed to a minimum of 30°: Position the adolescent in Fowler's position (sitting up at a 90-degree angle) or elevate the head of the bed to at least 30 degrees. This position helps prevent aspiration and facilitates the movement of the formula into the stomach.
7. Auscultate for bowel sounds and monitor tube placement: Use a stethoscope to auscultate the abdomen for bowel sounds. Absence of bowel sounds may indicate potential complications. Additionally, verify the placement of the nasogastric tube by checking for carbon dioxide detection, X-ray confirmation, or following facility-specific protocols.
8. Check gastric contents for pH: Aspirate a small amount of gastric contents using a syringe. Test the pH of the aspirate using pH strips or a pH meter. A pH range between 0 and 4 is considered a good indication of appropriate placement within the stomach.
9. Aspirate for residual volume: To assess gastric residual volume, use a syringe to withdraw the contents of the stomach through the nasogastric tube. Note the amount and appearance of the aspirate, as excessive residual volume may indicate feeding intolerance or delayed gastric emptying.
10. Flush the tubing with at least 30 mL of tap water: After checking gastric residual volume, flush the nasogastric tube with a minimum of 30 mL of tap water to ensure patency and prevent tube occlusion.
11. Administer the formula: Begin the enteral feeding by connecting the tubing to the nasogastric tube and initiating the flow of the formula according to the prescribed rate and schedule. Monitor the client's response during the feeding for any signs of intolerance or complications.
Throughout the procedure, adhere to proper infection control practices, maintain open communication with the adolescent and their family, and provide appropriate education and support.
To know more about incubation and enteral feeding visit:
https://brainly.com/question/15739559
#SPJ11
a solid takes up a definite amount of space true or flase
Answers
unu
nuunu
nnjbuboilujbn,mujjhhboñibhbbkbhboinbik
k
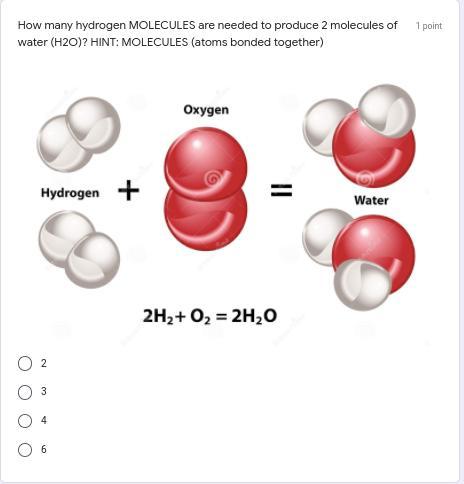
How many hydrogen MOLECULES are needed to produce 2 molecules of water (H2O)?
Answers
Sodium combines with water to produce sodium hydroxide and hydrogen gas. Which word equation represents this violent reaction?
Answers
The word equation that represents the violent reaction between sodium and water to produce sodium hydroxide and hydrogen gas is:
Sodium + Water → Sodium Hydroxide + Hydrogen
What is chemical equation ?The reactants and products involved in a chemical reaction, as well as their relative amounts, are shown in a chemical equation which is a symbolic representation of the process.
Therefore, The hydrogen gas could ignite and burst as a result of this reaction's strong exothermicity and potential heat release. As a result, it's crucial to handle sodium with caution and carry out this response in a controlled setting.
Learn more about chemical equation here : brainly.com/question/30265799
#SPJ1
diamonds are expensive and water is cheap. yet water is essential for life but diamonds are not essential to live. what explains this?
Answers
Water is inexpensive compared to diamonds. However, whereas water is necessary for life, diamonds are not. What causes this? The statements are all true.
Diamond is a mineral made primarily of carbon. It is the most well-known gemstone and also the hardest known naturally produced substance. If you want to test a loose gem, fill a standard glass with water about 3/4 of the way. Drop this stone into the glass with care. The diamond is real if it sinks to the bottom. It's probably a fake if it floats just at surface or slightly below. Place the stone with in water-filled glass. A it was will float when thrown into water because the higher densities of gems.
Learn more about diamonds here:
https://brainly.com/question/29775108
#SPJ4
copper forms two oxides. On heating 1 g of each in hydrogen 0.888 g and 0.798 g of the metal was obtained. Show that the results are in agreement with the law of multiple proportions.
Answers
Answer:
For the first oxide, 1 g gives 0.888 g of copper.
Dividing by 0.888 tells us that 1.126 g gives 1 g of copper so has 0.126 g of oxygen.
For the second oxide, 1 g gives 0.798 g of copper.
Dividing by 0.798 tells us that 1.253 g gives 1 g of copper so has 0.253 g of oxygen.
So 1 g of copper combines with either 0.126 g or 0.253 g of oxygen.
Within the limits of experimental error, 0.253 is twice 0.126, confirming the law of multiple proportion.
Use the following equation to answer the question below. How many moles of iron are made from 8.25 moles of Fe2O3?(see pic for equation)

Answers
Explanation: Using stoichiometry you can see that 1 Mol Fe2O3 becomes 2 Mol of Fe so, 8.25 is multiplied by 2.
Suppose an atom has 5 electrons, what is the total negative charge of the atom?
pls help
brainliest!!!
Answers
Answer:
Brainliest?
Explanation:
The total negative charge of an atom with 5 electrons is 5 times the charge of a single electron, which is -1.6 x 10^-19 coulombs.
So, the total negative charge of the atom is:
5 electrons x (-1.6 x 10^-19 C/electron) = -8 x 10^-19 C
Write the test for the following Gases.
Co2, H2,Cl2, Br2,
SO2,
NO2,
Answers
Answer:
oh no!! I can't do this ;(
Explanation:
oopsie "BrO" I can't do this pleaseee.
don't be selfish next time and maybe people will help you...
PLEASE HELP !
Convert 0.429 mol AlCl3 to grams

Answers
Answer:
asdd
Explanation:
adsade4223
Which best describes two ways in which hydrogen is involved in photosynthesis?
O1-Hydrogen is stored in leaves of plants.
2-Hydrogen combines with carbon dioxide to make glucose.
O1-Hydrogen is stored in leaves of plants.
2- Hydrogen combines with oxygen to make water.
O 1-Hydrogen enters plants as a component of water.
2-Hydrogen combines with carbon dioxide to make glucose.
O1-Hydrogen enters plants as a component of water.
2- Hydrogen combines with oxygen to make carbon dioxide.
Answers
Answer:
1- Hydrogen enters plants as a component of water.
2-Hydrogen combines with carbon dioxide to make glucose.
Explanation:
Hydrogen enters the plant bonded with Oxygen as water (H2O).
The water molecule splits and the Hydrogen combines with carbon dioxide to make glucose.
Answer:
1 - Hydrogen enters plants as a component of water.
2 - Hydrogen combines with carbon dioxide to make glucose.
Explanation:
what is carnal election?
Answers
Carnal election is a theological concept used in some Christian denominations, particularly in Calvinism.
It refers to the belief that God has chosen certain individuals for salvation based on His sovereign will and decision, and that this choice is not influenced by any action or merit of the individuals themselves.
This belief is in contrast to the idea of conditional election, in which God chooses individuals for salvation based on their faith or obedience. Carnal election is considered a cornerstone of Calvinist theology and is one of the key tenets of the Reformed tradition.
However, it is also a controversial and divisive issue, with some Christians rejecting it as incompatible with the idea of free will or with the character of a loving God.
To know more about sovereign click on the link below:
https://brainly.com/question/10867010#
#SPJ11
3.6 x 10^-3/2.1 x 10^6= 1.7 x 10^9
Answers
Answer:
(3.6×10^(-3))/(2.1×10^6) = 1.7×10^9 = False
Explanation:
Briefly explain how a titration experiment can be used to determine the concentration of a strong acid or strong base solution?
Answers
Answer:During an acid-base titration, an acid with a known concentration is slowly added to a base with an unknown concentration (or vice versa). A few drops of indicator solution are added to the base. The indicator will signal, by color change, when the base has been neutralizedExplanation:
What observation about baking powder suggests that it is a mixture of substances
Answers
When water is added to both baking powder and baking soda, baking powder reacts with water to produce a gas but baking soda does not produce any gas suggesting that it is a mixture of substances.
What is baking powder?Baking powder is mixture of substances which is used in baking.
Baking powder produces a gas, carbon dioxide which is required in leavening of baking products as well as to provide smoothness and consistency in the baked products.
Baking powder is a mixture of three ingredients namely; baking soda, cream of tartar, and cornstarch.
To show that baking powder is a mixture of substances, water is added to both baking powder and baking soda. It will be observed that baking powder reacts with water to produce a gas but baking soda does not produce any gas.
In conclusion, the different observations seen when water is added to baking powder and baking soda proves that baking powder is a mixture of substances.
Learn ore about baking powder at: https://brainly.com/question/20628766
#SPJ1
The solid, liquid, and gas states of water differ from each other in
Ο Α.
the mass of the individual molecules.
O B.
the size of the individual molecules.
Ос.
the average speed of the molecules.
O D.
the electrical charge of the molecules.
Answers
Answer:
B
Explanation:
the size of the individual molecules.
Explain your reasoning to why the rusting of iron is a chemical change (evidence not needed but may add it)
Answers
Answer:
A chemical change is when a new substance forms, which aligns with the rusting of iron since the iron is producing a different kind of matter
The percent composition of nh42c2o4
Answers
Answer: Percent composition by element
Element Symbol Mass Percent
Hydrogen H 6.498%
Carbon C 19.357%
Nitrogen N 22.574%
Oxygen O 51.571%
HOPE THIS HELPS
Which of the following is a form of stored energy?
A) heat energy
B) kinetic energy
C) sound energy
D) chemical potential energy
Answers
Answer:
option d
Explanation:
chemical potential energy
Answer:
D
Explanation:
Chemical potential energy is a form of stored energy. It is the potential energy between the chemical bonds of a substance.
how can coffee be turned back to water
Answers
Answer:
distillation, collecting and cooling the steam
Explanation: