Pure ethylenediamine(ED), H2NCH2CH2NH2 , is a liquid with a density of 0.896 g/ml. What mass of this compound would be needed to prepare 1.00 liter of a 0.030 M solution? What volume of pure ED would this mass correspond to?
Answers
2.012 ml of pure ED is needed to prepare 1.00 liter of a 0.030 M solution.To find the mass of ethylenediamine (ED) needed to prepare 1.00 liter of a 0.030 M solution, we can use the formula:
Molarity = moles of solute / volume of solution in liters
Rearranging the formula to solve for moles of solute:
moles of solute = molarity x volume of solution in liters
Plugging in the given values:
moles of solute = 0.030 M x 1.00 L = 0.030 moles
Now, we can use the molar mass of ED to find the mass needed:
mass of solute = moles of solute x molar mass of solute
The molar mass of ED is 60.10 g/mol. Plugging in the values:
mass of solute = 0.030 moles x 60.10 g/mol = 1.803 g
So, 1.803 g of ED is needed to prepare 1.00 liter of a 0.030 M solution.
To find the volume of pure ED that this mass corresponds to, we can use the density formula:
Density = mass / volume
Rearranging the formula to solve for volume:
Volume = mass / density
Plugging in the values:
Volume = 1.803 g / 0.896 g/ml
Volume = 2.012 ml
So, 2.012 ml of pure ED is needed to prepare 1.00 liter of a 0.030 M solution.
To know more about ethylenediamine refer here:
https://brainly.com/question/18008168
#SPJ11
Related Questions
What is the name of C3br8
Answers
Answer:
Octabromopropane or PubChem
PLEASE PEOPLE PLEEEEEASEEEREEE PEOPLE HEEEELPPPPP CAN YOU GIVE ME THE ANSWER!!! :,(

Answers
Answer:
its the middle one
Explanation:
the suns rays bounce off the planet easier at a lower angle.
Answer:
the middle option would heat up the least.
Explanation:
edge 2020, will give brainliest
1. The stream of electrically charged particles collide with gas atoms, causing the latter to give off light.
2. The electrically charged particles flow outward in all directions as far as Saturn.
3. The stream of electrically charged particles flows outward from the corona.
4. The particles of the solar wind flow along lines of the magnetic field.
Which shows the correct sequence in the formation of solar winds?
1, 2, 3, 4
2, 3, 4, 1
3, 2, 4, 1
4, 2, 1, 3
Answers
Answer:
3, 2, 4, 1
Explanation:
That's the correct sequence
A solution with a pH of 2 is how many timesmore acidic as a solution with a pH of 4?a. 2b. 0.5c. 1000d. 100e. 6
Answers
Solution with a pH of 2 is 100 times more acidic than a solution with a pH of 4. The correct answer is d. 100.
Find timesmore acidic as a solution with a pH of 4?A solution with a pH of 2 is how many times more acidic as a solution with a pH of 4?
To determine this, follow these steps:
Step 1: Calculate the difference in pH levels.
Difference = pH of 4 - pH of 2 = 4 - 2 = 2
Step 2: Use the formula for comparing acidity levels.
Acidity Ratio = 10^(Difference) = 10⁻²
Step 3: Find the answer.
Acidity Ratio = 100
Solution with a pH of 2 is 100 times more acidic than a solution with a pH of 4. The correct answer is d. 100.
Learn more about Timesmore acidic
brainly.com/question/29026766
#SPJ11
How many moles of each element (C, H, and O) are present in a 100.0 g sample of
ethanol?
Answers
Answer:
2.173 moles of ethanol is presented in a 100.0g sample of ethanol .
Explanation:
The amount of substance that contains as many Particles as there are atoms in exactly 12g of carbon- '12 isotope is called 1 mole '= 46 u.
(b) Draw a dot and cross diagram to show the formation of calcium oxide from atoms of
[2]
calcium and oxygen.
Answers
When calcium and oxygen interact, calcium oxide is created. Calcium oxide has the chemical formula CaO. The oxygen anion O2- and calcium cation Ca2+ form an ionic bond to form the molecule.
Which chemical is created when an O2 ion and a Ca2+ ion combine?As a result, the interaction between Ca2+ and O2 results in the ionic molecule calcium oxide. The chemical name is CaO.
How is calcium oxide produced from calcium?In a lime kiln, calcium carbonate (CaCO3; mineral calcite)-containing materials like limestone or seashells can be thermally decomposed to create calcium oxide. Calcination is the name of the method used to manufacture burnt lime.
To know more about Calcium oxide visit:-
brainly.com/question/20417380
#SPJ1
why is it important to increase the amount of solvent used if a reaction or purification procedure is scaled up?
Answers
Answer:
For the most part, how much a compound you can break up in a particular dissolvable is restricted. Sooner or later the arrangement becomes soaked. This intends that assuming you add a greater amount of the compound, it won't break up any longer and will stay strong all things considered.The term 'solvable' is applied to countless compound substances which are utilized to break down or weaken different substances or materials. They are generally natural fluids. Numerous solvents are likewise utilized as synthetic intermediates, fills, and as parts of a great many items.
Increase endeavors imply examination concerning potential cycle perils, figuring out response energy and thermodynamics, recognizing and describing contaminations, blending and mass exchange studies, heat move and intensity evacuation concentrates as well as crystallization and polymorph control.
Explanation:
It is relatively more noteworthy in sum than the solute. Like impetuses, a few solvents don't partake in synthetic responses. They just act as the response medium to empower synthetic responses to happen all the more quickly
It's critical that the two solvents are immiscible, on the grounds that then it is not difficult to isolate them from one another. The top fluid can be drawn off with a pipet, or the base layer can be depleted out by means of a stopcock. The mixtures that disintegrated in the ether have accordingly been isolated from the water-dissolvable mixtures.
You must add the base measure of bubbling dissolvable to get an immersed arrangement. On the off chance that you add a lot of dissolvable, the arrangement might be excessively weaken for gems to shape.
Specialized periodicals in which scientists publish the results of their works are called
Answers
Specialized periodicals in which scientists publish the results of their works are called scientific journals.
In educational publishing, a scientific journal is a periodical book intended to similarly the progress of technology, typically by way of reporting new studies.
Journal articles may include original research, re-analyses of studies, opinions of literature in a selected place, proposals of new but untested theories, or opinion pieces.
These scientific journals include the following.
original articles, case reports, technical notes, pictorial essays, reviews, commentaries editorials.Learn more about scientific journals here https://brainly.com/question/14443228
#SPJ10
Acids containing polyatomic ions are named according to the name of the anion. true or false
Answers
Answer:
true
Explanation:
Names for such acids consist of the prefix “hydro-“, the first syllable of the anion, and the suffix “-ic”. Complex acid compounds have oxygen in them. For an acid with a polyatomic ion, the suffix “-ate” from the ion is replaced with “-ic.”
•If I have 500 mL of vinegar and it contains 50. 4 g of acetic acid (MW = 60. 052 g/mol), what is the concentration of acetic acid in units of molarity?
Answers
The concentration of acetic acid in the vinegar solution is approximately 1.678 M.
To calculate the concentration of acetic acid (CH3COOH) in units of molarity (M), we need to use the formula:
Molarity (M) = Moles of solute / Volume of solution (in liters)
First, let's calculate the moles of acetic acid (CH3COOH) using its molar mass:
Molar mass of acetic acid (CH3COOH) = 60.052 g/mol
Mass of acetic acid = 50.4 g
Moles of acetic acid = Mass / Molar mass = 50.4 g / 60.052 g/mol = 0.839 mol
Next, we need to convert the volume of the solution from milliliters (mL) to liters (L):
Volume of solution = 500 mL = 500/1000 L = 0.5 L
Now, we can substitute the values into the formula to calculate the molarity:
Molarity = Moles of solute / Volume of solution = 0.839 mol / 0.5 L = 1.678 M
Therefore, the concentration of acetic acid in the vinegar solution is approximately 1.678 M.
To know more about concentration click this link -
brainly.com/question/3045247
#SPJ11
Does the diagram below demonstrate an endothermic or an exothermic reaction? Explain your reasoning.
PLEASE BE ACCURATE!!! Thank you so much!!:))
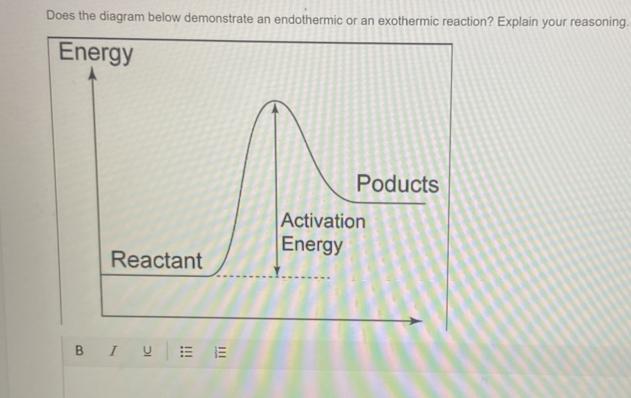
Answers
Answer:
Endothermic reaction.
Explanation:
The reactants are at a lower energy rate than the products. Because delta H is positive, energy is absorbed from the surroundings.
A student dissolves 13. g of stilbene (C14H12) in 100. mL of a solvent with a density of 0.85 g/mL. The student notices that the volume of the solvent does not change when the stilbene dissolves in it.
Calculate the molarity and molality of the student's solution. Be sure each of your answer entries has the correct number of significant digits.
Answers
Answer:
Molarity = 0.72M
Molality = 0.85M
Explanation:
Molarity and molality are two measures of molar concentration, and they can be calculated as follows:
Molarity = number of moles/volume of solvent
Molality = number of moles/mass (kg) of solvent
Mole = mass/molar mass
Molar mass of stilbene (C14H12) = 12(14) + 1(12)
= 168 + 12
= 180g/mol
mole of stilbene = 13/180
mole = 0.072mol
Volume = 100mL = 0.1L
molarity = 0.072/0.1
Molarity = 0.72M
Molality = number of moles ÷ mass (kg) of solvent
To get mass of solvent, we use;
Density = mass/volume
Mass = density × volume
Mass = 0.85g/mL × 100mL
Mass = 85g
Mass in kg = 85/1000 = 0.085kg
Molality = 0.072/0.085
Molality = 0.847M
Molality = 0.85M
How the Bohr model explains both of these observations
Answers
The Bohr model explains the observations by suggesting that electrons exist in specific energy levels and transitions between these levels cause the observed colors.
The Bohr model of an atom explains the observations of line spectra and quantized energy levels. Line spectra is a phenomenon where atoms emit or absorb light at specific wavelengths. Quantized energy levels refer to the specific energies that electrons can possess while occupying specific energy levels.
The Bohr model explains both of these observations by proposing that electrons can only exist in specific energy levels and can move between them by absorbing or emitting photons of specific energies. An electron in an atom can exist only in one of the allowed energy levels.
These energy levels are defined by the Bohr radius formula:
\(r(n) = n^2 * h^2 / 4\)π\(^2mke^2\)
Where r(n) is the radius of the nth energy level, n is an integer representing the energy level, h is Planck's constant, m is the mass of the electron, ke is Coulomb's constant, and e is the charge of the electron.Electrons emit light when they move from a higher energy level to a lower one and absorb light when they move from a lower energy level to a higher one.
The energy of the photon emitted or absorbed is equal to the difference in energy between the two levels. This explains why line spectra occur, as each atom emits or absorbs light at specific wavelengths corresponding to the energy difference between its allowed energy levels.The Bohr model's proposal of quantized energy levels provides an explanation for the stability of atoms. Electrons in an atom can't exist between energy levels, so they can't radiate energy and spiral into the nucleus.
Know more about Bohr model here:
https://brainly.com/question/29400473
#SPJ8
____________ is the process used to separate the solvent from a solution
choices-
Distillation
Hydration
oxidation
All the above
Answers
:) :) :) :) :) :)
What do you expect to see in a test tube when several drops of two aqueous solutions are mixed, and an insoluble precipitate is formed?.
Answers
When we mix several drops of two aqueous solutions and an insoluble precipitate is formed then we may expect to see a cloudy appearance.
In performing chemical reactions when some quantity of two solutions are mixed together and formation of a solid form of residue takes place beneath the liquid, then such reactions are known as precipitation reactions. In double displacement reaction between two different solutions, the insoluble salts separates out to form precipitate. For example in the reaction between sodium hydroxide and copper sulphate, copper hydroxide gives a pale blue precipitate.
2NaOH(aq) + CuSO₄(aq) → Cu(OH)₂ (s) + Na₂SO₄ (aq)
pale blue ppt
Hence, these insoluble precipitates gives a cloudy appearance to the solution. They are mostly coloured or white.
To know more about precipitate here
https://brainly.com/question/25505239
#SPJ1
Atmospheric pressure on the peak of Mt. Everest can be as low as 150 mm
Hg, which is why climbers need to bring oxygen tanks for the last part of
the climb. If the climbers carry 10.0 liter tanks with an internal gas
pressure of 30400 mm Hg, what will be the volume of the gas when it is
released from the tanks?
Answers
Answer:
Volume of gas when release form tank = V₂ = 2026.67 L
Explanation:
Given data:
Initial volume of gas =V₁= 10.0 L
Pressure of gas inside tank =P₁= 30400 mmHg
Volume of gas when release form tank =V₂= ?
Pressure at peak =P₂= 150 mmHg
Solution:
The given problem will be solved through the Boly's law,
"The volume of given amount of gas is inversely proportional to its pressure by keeping the temperature and number of moles constant"
Mathematical expression:
P₁V₁ = P₂V₂
P₁ = Initial pressure
V₁ = initial volume
P₂ = final pressure
V₂ = final volume
Now we will put the values in formula,
P₁V₁ = P₂V₂
30400 mmHg × 10.0 L = 150 mmHg × V₂
V₂ = 304000 mmHg. L/ 150 mmHg
V₂ = 2026.67 L
Which best describes the relationship between the circulatory system and the respiratory system?
The circulatory system is the transport system for the respiratory system.
The respiratory system is the transport system for the circulatory system.
The circulatory system diffuses red blood cells into the respiratory system to pick up oxygen.
The respiratory system transfers hemoglobin to the red blood cells of the circulatory system.
Answers
Answer:
its a
Explanation:
yw
The respiratory and circulatory systems work together to maintain the homeostasis of the human body. The circulatory system is the transport system for the respiratory system. The correct option is A.
What is respiratory system?The network of organs and tissues which help us to breathe is defined as the human respiratory system. The main function of this system is to introduce oxygen into the body and to expel carbon dioxide from the body.
The human circulatory system consisting of arteries, veins and capillaries provide all the essential nutrients, minerals and hormones to the various parts of the body.
The respiratory system does not work alone in transporting oxygen through the body. The oxygen taken in from the respiratory system moves into blood vessels that then circulate oxygen rich blood to tissues and cells.
Thus the correct option is A.
To know more about the respiratory system, visit;
https://brainly.com/question/19306828
#SPJ3
Notes for timeline panel
Answers
What is the product of the reaction between
Aluminum and lodine?
12 (iodine gas)?
Answers
Answers:
Aluminium Iodide
(AlI³)

Methane (CH4) is a gas that is found in small quantities in Earth’s atmosphere. Which type of bonds does methane have, and why does one carbon atom bond with four hydrogen atoms? In three to five sentences, explain your answer in terms of valence electrons and electronegativity.
Answers
In methane, the covalent bond is present between one carbon and four hydrogen atoms.
What is methane?Methane is the simplest form of saturated hydrocarbons. Methane is an odorless gas as well as a colorless gas. It has one carbon and four hydrogen atoms which is why methane is also called a tetrahedral molecule.
CH₄ is the chemical formula of methane and is a non-toxic but flammable gas. From the tetrahedral structure of methane, we can see that a carbon atom is bonded to four hydrogen atoms.
One molecule of methane contains four covalent bonds. Each covalent bond is formed between the carbon and one hydrogen atom. The formation of a covalent bond between carbon and hydrogen is due to the small electronegativity difference between carbon and hydrogen.
In this way, carbon completes its octet and hydrogen completes its duplet. Therefore, the covalent bond is formed between one carbon atom bond with four hydrogen atoms in methane.
Learn more about methane, here:
https://brainly.com/question/2127750
#SPJ2
Shelby measured the volume of a cylinder and determined it to be 54.5 cm3 . The teacher told her that she was 4.25% too high in her determination of the volume. What is the actual volume of the cylinder
Answers
Answer:
V = 56.816 cm³
Explanation:
Given that,
The measured value of the cylinder is 54.5 cm³
The percent error in the measurement of the volume is 4.25%
We need to find the actual volume of the cylinder.
Firstly, we can find the error in the calculation as follows :
\(E=54.5 \times \dfrac{4.25}{100}=\pm2.316\)
It is mentioned in the problem that, the teacher told her that she was 4.25% too high in her determination of the volume. It would mean that,
Total volume = 54.5 + 2.316 = 56.816 cm³
Hence, the actual volume of the cylinder is 56.816 cm³.
The volume of a sample of oxygen is 300.0 mL when the pressure is 1.00 atm and the temperature is 27.0°C. At what temperature is the volume 1.00 L and the pressure 0.500 atm?
Answers
A π bond could be formed from the overlap of which two orbitals? A) two sp² hybrid orbitals B) a 1s and a sp² hybrid orbital C) a sp and a sp² hybrid orbitals D) two unhybridized p orbitals
Answers
Answer:
D) two unhybridized p orbitals
Explanation:
In covalent bond, to form a bond, each of the two participating atoms would put down an unpaired electron to be used in forming a shared pair of electrons between them.
There are two types of covalent bonds:
A sigma bond \(\sigma\)
A pi bond \(\pi\)
A sigma bond is formed when a hybrid orbital (sp,sp² and sp³) overlaps with another hybrid orbital or with s- or p- orbital.
A pi bond is formed when a p-orbital overlaps with another parallel p-orbital laterally. This implies that ,a π bond could be formed from the overlap of two unhybridized p orbitals.
HELP MEE PLEASEE Marco put a pot of water on to boil eggs. After a few minutes, all the water was gone. This is an example of what?
Answers
Answer:
Evaporation
Explanation:
The water evaporated when heat was added causing the water to go.
Answer:
When water is heated the molecules move faster and since they vibrate the evaporate.
Explanation:
sciense :)
Describe how to prepare 100 mL of a 5 M hydrochloric acid solution from concentrated HCl (12.1M).
Answers
To prepare 100 mL of a 5 M hydrochloric acid solution from concentrated HCl (12.1 M), you will need to dilute the concentrated HCl. You will need to calculate the amount of concentrated HCl needed to make the solution.
To prepare 100 mL of a 5 M hydrochloric acid solution from concentrated HCl (12.1 M), follow these steps:
1. Determine the amount of concentrated HCl needed using the dilution equation: M1V1 = M2V2. Here, M1 is the initial concentration (12.1 M), V1 is the volume of concentrated HCl needed, M2 is the final concentration (5 M), and V2 is the final volume (100 mL).
2. Plug in the known values into the equation: (12.1 M)(V1) = (5 M)(100 mL).
3. Solve for V1: V1 = (5 M × 100 mL) / 12.1 M ≈ 41.3 mL.
4. Measure out 41.3 mL of concentrated HCl using a graduated cylinder or pipette.
5. Add the 41.3 mL of concentrated HCl to a volumetric flask or beaker.
6. Gradually add distilled water to the flask or beaker containing the concentrated HCl, stirring gently, until the total volume reaches 100 mL. This will dilute the concentrated HCl to a 5 M solution.
7. Make sure to properly label the container holding the 5 M hydrochloric acid solution.
By following these steps, you will have successfully prepared 100 mL of a 5 M hydrochloric acid solution from concentrated HCl (12.1 M).
Learn more about 5 M hydrochloric acids at https://brainly.com/question/27576431
#SPJ11
What is a periodic trend?
O A. An older version of the periodic table
O B. A pattern that appears on the periodic table
C. Any characteristic of the noble gases
O D. A numbering system for the periodic table
Answers
Answer:
b
Explanation:
when a phase change occurs the temperature of the matter will ____________ until the phase change is complete.
Answers
When a phase change occurs, the temperature of the matter will remain constant until the phase change is complete.
This is because the energy that is added to or removed from the matter during a phase change is used to either break or form intermolecular bonds between the particles in the substance, rather than to increase or decrease the kinetic energy of the particles.
For example, during the melting of ice, the temperature of the ice remains constant at 0 degrees Celsius until all of the ice has melted. Similarly, during the boiling of water, the temperature of the water remains constant at 100 degrees Celsius until all of the water has boiled off.
Once the phase change is complete, the temperature of the matter will either increase or decrease again, depending on whether heat is being added to or removed from the substance. This is because the kinetic energy of the particles in the substance is once again affected by the addition or removal of heat.
To know more about phase change here
https://brainly.com/question/30270780
#SPJ4
the proton concentrations of three solutions at 25 °c are given. classify the solutions as acidic, basic, or neutral.
Answers
This problem is giving information about the proton concentrations of three solutions at 25 °C. Despite they are not numerically given, we can propose three scenarios to see how to approach the question.
Let the following solutions to come up:
[H⁺] = 2.63x10⁻³ M
[H⁺] = 1.00x10⁻⁷ M
[H⁺] = 4.511x10⁻⁹ M
The first step, will be the calculation of the pH for each solution via:
pH = -log([H⁺])
So that they turn out to be:
pH = -log(2.63x10⁻³ M) = 2.580
pH = -log(1.00x10⁻⁷ M) = 7.000
pH = -log(4.511x10⁻⁹ M) = 8.3457
In such a way, since acidic solutions have a pH below 7, neutral have a pH equal to 7 and basic have it above 7, we infer the first one is acidic, second one is neutral and third one is basic.
Thus, you can reproduce this methodology with the proton concentrations you are given.
Learn more:
https://brainly.com/question/23659500https://brainly.com/question/23428840The vapor pressure of benzene is 53.3 kPa at 60.6 °C, but it fell to 51.4 kPa when
20.3 g of a non-volatile organic compound was dissolved in 500 g of benzene. The molar weight of benzene is 78.11 g/mol. Calculate the molar mass of the compound. Assume that the solution is ideal and follows Raoult’s law.
Answers
Assuming that the solution is ideal and follows Raoult’s law, the molar mass of the compound is 16.8 g/mol.
To calculate the molar mass of the compound, and we are to assume that the solution is ideal and follows Raoult’s law. According to Raoult’s law, the partial vapor pressure of a component of an ideal solution is proportional to its mole fraction.
Mole fraction is the ratio of the number of moles of a particular component to the total number of moles of the solution. It is given as:
Total number of moles of the solution = Number of moles of the first component + Number of moles of the second component + …. + Number of moles of the nth component.
Mole fraction of the first component = Number of moles of the first component/Total number of moles of the solution.
Mole fraction of the second component = Number of moles of the second component/Total number of moles of the solution.
Mole fraction of the nth component = Number of moles of the nth component/Total number of moles of the solution.
So, we know that the vapor pressure of the solution is equal to the sum of the vapor pressures of each component of the solution. Therefore, we can write:
Psolution = P1 + P2 + P3 + …… + Pn
Here, P1, P2, P3, ….. , Pn are the vapor pressures of the components of the solution. And, Psolution is the vapor pressure of the solution.
Mass of the solute (Non-volatile organic compound) = 20.3 g
Mass of the solvent (Benzene) = 500 g
Vapor pressure of pure benzene at 60.6 °C = 53.3 kPa
Vapor pressure of the solution = 51.4 kPa
Molar mass of benzene = 78.11 g/mol
We can find the mole fraction of benzene and the solute as:
Mass of benzene = 500 g
Molar mass of benzene = 78.11 g/mol
Number of moles of benzene = mass/molar mass = 500/78.11 = 6.399 mol
Mole fraction of benzene = Number of moles of benzene/Total number of moles of the solution = 6.399/(6.399 + Number of moles of the solute)
We know that, according to Raoult’s law:
P1 = (Mole fraction of benzene) × (Vapor pressure of pure benzene at 60.6 °C)
P2 = (Mole fraction of the solute) × 0 [Because the compound is non-volatile.]
Psolution = P1 + P2
Psolution = (Mole fraction of benzene) × (Vapor pressure of pure benzene at 60.6 °C) + 0.0 = (6.399/(6.399 + Number of moles of the solute)) × (53.3 kPa)
The vapor pressure of the solution is given as 51.4 kPa. Hence, we can write:
(6.399/(6.399 + Number of moles of the solute)) × (53.3 kPa) = 51.4 kPa
By solving the above equation, we can find the number of moles of the solute.
Number of moles of the solute = 1.205 mol
Molar mass of the solute can be calculated as follows:
Molar mass of the solute = Mass of the solute/Number of moles of the solute = 20.3 g/1.205 mol = 16.8 g/mol
Therefore, the molar mass of the compound is 16.8 g/mol.
Learn more about Raoult’s law here: https://brainly.com/question/28304759
#SPJ11
True or False: The relative concentrations of ATP and ADP control the cellular rates of the citric acid cycle
Answers
True. The relative concentrations of ATP and ADP play a critical role in controlling the cellular rates of the citric acid cycle, also known as the Krebs cycle or the tricarboxylic acid (TCA) cycle.
The citric acid cycle is a series of reactions that occur in the mitochondria and generates energy by oxidizing acetyl-CoA. This energy is stored in the form of ATP, which is used to power various cellular processes.
When the cellular ATP levels are high, the rate of the citric acid cycle slows down because the cell has sufficient energy and does not need to produce more. On the other hand, when the cellular ATP levels are low, the rate of the citric acid cycle increases to produce more ATP.
Learn more about citric acid cycle here:
https://brainly.com/question/29857075
#SPJ11