Answers
Answer:
Y
Explanation:
i think the answer is y because it is down hill and x is where the energy is let out from.
Answer:
Y
Explanation:
cause when the Z side of the rollercoaster, the fastest one goes with the Y and the Y stops it because it's too heavy or it's too fast :|
Related Questions
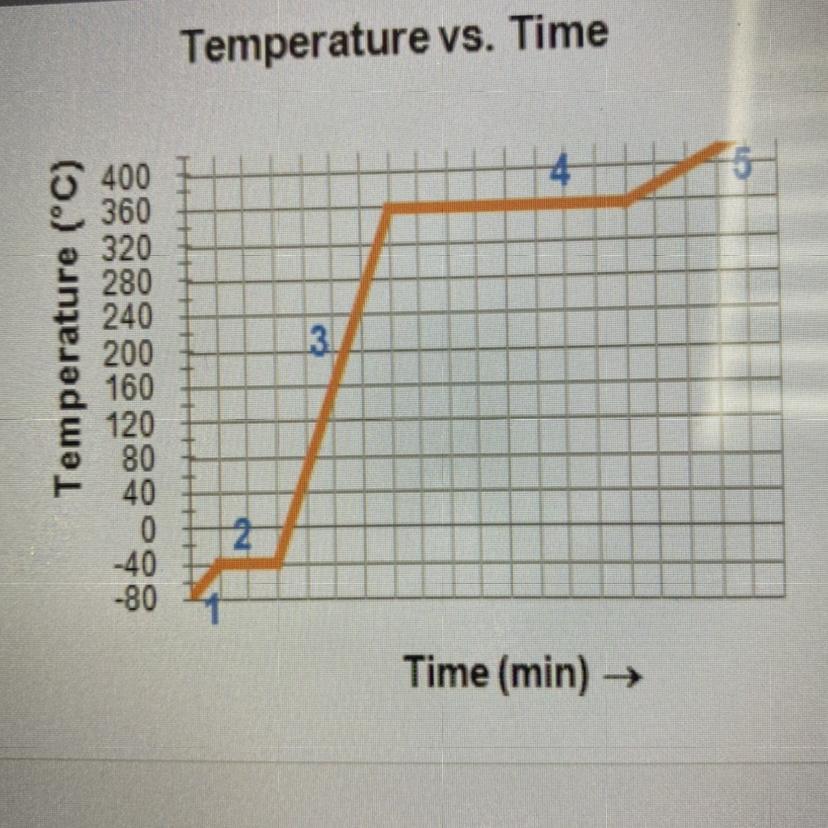
Complete the statements by writing the number
from the graph.
The substance is in the gas phase only in region
The substance is in both the liquid and the solid
phase in region
The substance is in only the liquid phase in region
The melting point is the temperature at region
The boiling point is the temperature at region
Answers
Answer :
The substance is in the gas phase only in region → 5
The substance is in both the liquid and the solid phase in region → 2
The substance is in only the liquid phase in region → 3
The melting point is the temperature at region → 2
The boiling point is the temperature at region → 4
Explanation :
Six phases of substance:
Melting or fusion : In this process the phase changes from solid state to liquid state at constant temperature.Freezing : In this process the phase changes from liquid state to solid state at constant temperature.Evaporation : In this process the phase changes from liquid state to gaseous state at constant temperature.Condensation : In this process the phase changes from gaseous state to liquid state at constant temperature.Sublimation : In this process the phase changes from solid state to gaseous state without passing through the liquid state at constant temperature.Deposition : In this process the phase changes from gaseous state to solid state without passing through the liquid state at constant temperature.Answer:
5, 2, 3, 2, 4.
Explanation:
got it correct on edge 2021
25 ml of 2.0 M silver acetate reacts with grams 35 ml of 1.0 M Calcium chloride
Answers
Answer:
\(m_{AgCl}=7.15gAgCl\\m_{Ca\ Acet}=3.15gCa\ Acet\\\)
Explanation:
Hello,
In this case, with the given information, we can identify the limiting reactant and compute the theoretical yield for the undergoing chemical reaction:
\(2CH_3COOAg+CaCl_2\rightarrow (CH_3COO)_2Ca+2AgCl\)
Thus, with the given concentrations and volumes we compute the available moles of silver acetate:
\(n_{Ag\ Acet}=2.0mol/L*0.025mL=0.05mol\)
Then, the moles of silver acetate that are consumed by 35 mL of 1.0 M calcium chloride:
\(n=0.035mL*1.0molCaCl_2/L*\frac{2mol Ag\ Acet}{1molCaCl_2} =0.07mol Ag\ Acet\)
Therefore, since there are less available moles, it is the limiting reactant, for that reason, the theoretical yields of both calcium acetate and silver acetate are:
\(m_{AgCl}=0.05mol Ag\ Acet*\frac{2molAgCl}{2mol Ag\ Acet} *\frac{143gAgCl}{1molAgCl} \\\\m_{AgCl}=7.15gAgCl\\\\m_{Ca\ Acet}=0.05mol Ag\ Acet*\frac{1molmol Ca\ Acet}{2molmol Ag\ Acet} *\frac{126gmol Ca\ Acet}{1mol Ca\ Acet} \\\\m_{Ca\ Acet}=3.15gCa\ Acet\)
Best regards.
Please help I’m really stuck:/
1. Use the equation weight = mg to find the weight of a 45 kg child.
2. Find the speed of a caterpillar that crawls a distance of 6.0 cm every
2.0 seconds. The equation for speed is v=d/t.
3. The circumference of a circle equals 2 mr, where r is the radius. Find
the circumference of a compact disc that has a radius of 6.0 cm.
2
HOLT SCIENCE SPECTRUM
Answers
Answer:
i would say two
Explanation:
I did the math
If the temperature is lowered on a ______________________________, a condensate will be produced.
Answers
If the temperature is lowered to a dew point, condensate will be produced.
While the air temperature drops underneath its dew factor, excess moisture can be released in the form of condensation. Condensation problems are maximumly probable to occur in climates in which temperatures regularly dip to 35°F or colder over a prolonged time period.
The dew factor is the temperature to which air needs to be cooled to emerge as saturated with water vapor, assuming steady air stress and water content. while cooled beneath the dew point, moisture potential is reduced and airborne water vapor will condense to form liquid water known as dew. Both the air is cooled to its dew point or it becomes so saturated with water vapor that it can't maintain any extra water. The dew factor is the temperature at which condensation occurs
Learn more about temperature here:-https://brainly.com/question/24746268
#SPJ9
All combustion reactions have oxygen as a reactant. (2 points)
Group of answer choices
True
False
Answers
Answer:
true
Explanation:
They all have a hydrocarbon plus oxygen.
The given statement that "all combustion reactions have oxygen as a reactant" is true. Combustion is a type of chemical reaction where a fuel (usually a hydrocarbon) combines with oxygen gas to produce heat, light, and new chemical compounds, such as carbon dioxide and water.
For combustion to occur, there must be a fuel source, oxygen, and a source of ignition, such as a spark or heat. Oxygen acts as a reactant because it combines with the fuel source to produce the new compounds. Without oxygen, the reaction cannot occur. It is important to note that not all reactions involving oxygen are combustion reactions. For example, rusting of iron is a reaction that involves oxygen, but it is not a combustion reaction.
In combustion reactions, the heat and light produced are often used for industrial processes, transportation, or heating. However, the reaction can also be destructive if not controlled, such as in wildfires or explosions. In conclusion, all combustion reactions have oxygen as a reactant, as it is necessary for the reaction to occur and produce the desired products.
For more such questions on combustion
https://brainly.com/question/13251946
#SPJ11
Define exothermic and endothermic. What are the mathematical signs of the internal energy and enthalpy when a process is exothermic?
Answers
Exothermic refers to chemical interactions that aerobic respiration. Combustion reactions release higher energy. Endothermic refers to atoms and molecules that either use or absorb reactive power.
What is an exothermic explanation?A chemical process known as an endothermic releases energy as heat or light. It is an endothermic reaction's opposite. Chemical equation expressed as reactants + products + energy. An reaction mechanism is one in which electricity is given off as light or warmth.
Exothermic example: What is it?A response is deemed to be exothermic if it produces heat while also undergoing a net decrease in basic enthalpy change. Samples include those type of combustion, iron rust, including water froze. Exothermic processes are those that discharge heat and energy into the surroundings.
To know more about exothermic visit:
https://brainly.com/question/13243759
#SPJ1
The isotope Ti-48 is produced by the alpha decay of which of the following:
a) ⁵³Mn
b) ⁵⁴Cr
c) ⁵³V
d) ⁵⁴V
e) ⁵²Cr
Answers
Answer:
e) ⁵²Cr
Explanation:
The general form of alpha decay is as follows:
\(\boxed{ ^A_ZX \ \ \rightarrow \ \ ^{A - 4} _{Z - 2} \ Y \ \ + \ \ ^4_2 \alpha}\).
From this, we can see that during alpha decay, the mass number decreases by 4 and the atomic number decreases by 2.
Therefore, we need to find a nucleus that has 4 more nucleons (i.e., a mass number that is 4 more) than that of Ti-48, which is 48 + 4 = 52.
The only option with a nuclear number of 52 is ⁵²Cr, and therefore, Ti-48 is produced by the alpha decay of ⁵²Cr.
What is the predicted change in the boiling point of water when 1.50 g of
barium chloride (BaCl₂) is dissolved in 1.50 kg of water?
Kb of water = 0.51°C/mol
molar mass BaCl₂ = 208.23 g/mol
ivalue of BaCl₂ = 3
Answers
Answer:
0.00735°C
Explanation:
By seeing the question, we can see the elevation in boiling point with addition of BaCl₂ in water
⠀
\( \textsf {While} \: \sf {\Delta T_b} \: \textsf{expression is used} \\ \textsf {for elevation of boiling point}\)
⠀
⠀
The elevation in boiling point is a phenomenon in which there is increase in boiling point in solution, when the particular type of solute is added to pure solvent.
⠀
⠀
\( \sf \large \underline{The \: formula \: to \: be \: used \: in \: this \: question \: is} \\ \boxed{T_b = i \times K_b \times m}\)
⠀
⠀
Where 'i' is van't hoff factor which represents the ratio of observed osmotic pressure and the value to be expected.
and 'i' is 3 (as given in the question)
⠀
'Kb' is molal boiling point constant. And it's value is 0.51°C/mol(given in question)
⠀
'm' represent the molality of solution. Molatity is no. of moles of solution present in 1kg of solution.
⠀
⠀
To find molality, we have to divide no. of moles of solute by weight of solution
⠀
While first we need to no. of moles
\( \sf \implies no. \: of \: moles = \frac{weight \: of \: solute}{molar \: mass \: of \: solute} \\ \\ \implies \sf no. \: of \: moles = \frac{1.5}{208.23} \\ \\ \sf \implies no. \: of \: moles = 0.0072 \)
⠀
⠀
Now, we will find molality
⠀
\( \sf \hookrightarrow molality = \frac{no.\: of \: moles}{weight \: of \: solution} \\ \\ \sf \hookrightarrow molality = \frac{0.072}{1.5} \\ \\ \sf \hookrightarrow molality = 0.048 \: mol {kg}^{ - 1} \)
⠀
⠀
\( \textsf{ \large{ \underline{Now substituting the required values}}} \)
⠀
\( \sf \longmapsto \Delta T_b = 3 \times 0.51 \times 0.0048 \\ \\ \\ \boxed{ \tt{ \longmapsto \Delta T_b =0.00735{ \degree}C}}\)
⠀
⠀
⠀
Henceforth, the change in boiling point is 0.00735°C.
Help plz :( Convert 48,000 seconds into hours. (Two step problem, use two conversion factors in a row)
Answers
Answer:
13.3 h
Explanation:
Step 1: Given data
Time (t): 48,000 seconds
Step 2: Convert "t" from seconds to minutes
We will use the conversion factor 1 min = 60 s.
48,000 s × 1 min/60 s = 800 min
Step 3: Convert "t" from minutes to hours
We will use the conversion factor 1 h = 60 min.
800 min × 1 h/60 min = 13.3 h
To sum up, 48,000 seconds is equal to 13.3 hours.
According to the Arrhenius equation, changing which factors will affect the
rate constant?
OA. The constant A and the temperature
B. Temperature and activation energy
C. Temperature and the ideal gas constant
D. The activation energy and the constant A
SUBMIT
Answers
The correct answer is B: Temperature and activation energy are the factors that affect the rate constant according to the Arrhenius equation.
According to the Arrhenius equation, which describes the relationship between the rate constant (k) of a chemical reaction and temperature, changing the factors of temperature and activation energy will affect the rate constant.
The Arrhenius equation is given by:
k = A * e^(-Ea/RT),
where k is the rate constant, A is the pre-exponential factor or the frequency factor, Ea is the activation energy, R is the ideal gas constant, T is the temperature in Kelvin, and e is the base of the natural logarithm.
From the equation, it is evident that the rate constant is directly influenced by temperature and activation energy. Increasing the temperature results in a higher rate constant, as the exponential term in the equation becomes larger. This is because higher temperatures provide more kinetic energy to the reacting molecules, leading to more frequent successful collisions and increased reaction rates.
Similarly, the activation energy affects the rate constant. A higher activation energy results in a lower rate constant, as the exponential term becomes smaller. Activation energy represents the energy barrier that reactant molecules must overcome to form products. A higher activation energy implies a slower reaction rate. option(B)
For such more questions on Temperature
https://brainly.com/question/30668924
#SPJ8
What is the theoretical yield of BOTH products when 100.0 g of Cr2O3 reacts with 100.0 g of C?
Answers
Theoretical yield of Cr is 68.43 g and the theoretical yield of CO2 is 1.1 kg when 100.0 g of Cr2O3 reacts with 100.0 g of C.
What is theoretical yield?Quantity of a product obtained from complete conversion of the limiting reactant in chemical reaction is called theoretical yield.
2 Cr2O3 + 3 C → 4 Cr + 3 CO2
Molar mass of Cr2O3 is 152 g/mol, and the molar mass of C is 12.01 g/mol. Therefore, 100.0 g of each reactant is equivalent to:
100.0 g Cr2O3 / 152 g/mol = 0.6579 mol Cr2O3
100.0 g C / 12.01 g/mol = 8.329 mol C
The stoichiometry of the balanced equation tells us that for every 2 moles of Cr2O3, we need 3 moles of C. Therefore, if we have 0.6579 mol of Cr2O3, we need (3/2) x 0.6579 = 0.9868 mol of C to react completely. However, we only have 8.329 mol of C, which is in excess. This means that Cr2O3 is limiting reactant, and C is excess reactant.
From the balanced equation, 2 moles of Cr2O3 produce 4 moles of Cr.
Therefore, 0.6579 mol of Cr2O3 will produce (4/2) x 0.6579 = 1.316 mol of Cr.
Molar mass of Cr is 52.0 g/mol, so the theoretical yield of Cr is 1.316 mol x 52.0 g/mol = 68.43 g.
Therefore, 8.329 mol of C will produce (3/1) x 8.329 = 24.99 mol of CO2.
Molar mass of CO2 is 44.01 g/mol, so the theoretical yield of CO2 is 24.99 mol x 44.01 g/mol = 1099.25 g or 1.1 kg.
Therefore, theoretical yield of Cr is 68.43 g and theoretical yield of CO2 is 1.1 kg when 100.0 g of Cr2O3 reacts with 100.0 g of C.
To know more about theoretical yield, refer
https://brainly.com/question/25996347
#SPJ1
While performing a neutralization reaction, Jonna added 27.55 mL of 0.144 M H2SO4 to 43.84 mL of 0.316 M KOH. How many moles of OH- are unreacted in the solution after the neutralization is complete?
Answers
Answer:
5.916x10⁻³ mol OH⁻
Explanation:
The reaction that takes place is:
H₂SO₄ + 2KOH → K₂SO₄ + 2H₂OFirst we calculate the added moles of each reagent, using the given volumes and concentrations:
H₂SO₄ ⇒ 0.144 M * 27.55 mL = 3.967 mmol H₂SO₄KOH ⇒ 0.316 M * 43.84 mL = 13.85 mmol KOHNow we calculate how many KOH moles reacted with 3.967 mmol H₂SO₄:
3.967 mmol H₂SO₄ * \(\frac{2mmolKOH}{1mmolH_2SO_4}\) = 7.934 mmol KOHFinally we calculate how many OH⁻ moles remained after the reaction
13.85 mmol - 7.934 mmol = 5.916 mmol OH⁻5.916 mmol / 1000 = 5.916x10⁻³ mol OH⁻sp3-orbitals
bent
tetrahedral
trigonal bipyramidal
Answers
The shape of the sp3 orbitals is tetrahedral.
What are orbitals?The term orbital refers to a region in space where there is a high probability of finding the electron. We know that sometimes orbitals could be combined in order to obtain the orbitals that are suited in energy to participate in chemical bonding. This is known as the hybridization of orbitals.
The sp3 orbital is a hybrid orbital The four hybrid orbitals are directed towards the corners of a regular tetrahedron. Hence the shape of the sp3 orbitals is tetrahedral.
Learn more about shapes of orbitals:https://brainly.com/question/11793076
#SPJ1
Answer:
sp2
bent
trigonal planar
sp3
bent
tetrahedral
Explanation:
18. Which equation represents an equilibrium system? 2Mg(s) + O₂(g) — 2MgO(s) O = CO₂ (s) CO₂(g) Agt (aq) + Cr (aq) — O 250₂(g) + O₂(g) — AgCl (s) 250, (g)
Answers
An equilibrium reaction occurs when the rates of forward and backward reactions are equal. Therefore, the correct equation representing an equilibrium system is:
2Mg(s) + O₂(g) ⇌ 2MgO(s)
An equilibrium reaction is a reversible reaction in which the rate of the forward reaction is equal to the rate of the backward reaction. It is a state of balance in which the concentrations of reactants and products remain constant over time.An equilibrium equation is a chemical reaction in which the forward and backward reactions are equal and there is no net change in the concentration of reactants or products. A reversible arrow (↔) indicates that the reaction is at equilibrium. The forward and reverse reactions occur at the same rate in an equilibrium reaction.For such more questions on equilibrium reaction
https://brainly.com/question/31383509
#SPJ8
Which correctly lists the three weather factors that are indicators of climate change?
a.) sunsets, wind patterns, clouds
b.) ocean currents, ice cores, temperature
c.) temperature, wind patterns, ice cores
d.)wind patterns, temperature, ocean currents
Answers
Answer:
C
Explanation:
c.) temperature, wind patterns, ice cores
How many moles are there in 3.4*10^26 moles of Ag
Answers
Answer:
It would be 151.832775 because one mole is 44.0095*3.45 i hope this helps!
Explanation:
Graphite and diamond are both solid forms of the element carbon. Which statement explains
the different properties of these two forms of carbon?
(1) Diamond has ionic bonding and graphite has metallic bonding.
(2) Diamond has metallic bonding and graphite has ionic bonding.
(3) Diamond has a different crystal structure from graphite.
(4) Diamond has carbon atoms with more valence electrons than graphite.
Answers
Answer: C diamond has a different crystal structure from graphite
Explanation:
The difference between the properties of two forms of carbon is that
Diamond has a crystal structure different from graphite.
Graphite and Diamond are known as allotropes of Carbon as they have the
same chemical properties but different crystal structure.
Diamonds form a 3 crystal lattice with no flexibility while Graphite are
bonded to sheets which makes them slide easily within one another and
gives it its soft texture.
Read more on https://brainly.com/question/23491824
Describe how moving the mountain lions could affect the ecosystem
Answers
Answer:
If Mountain lions are removed from a habitat/ecosystem, their prey will overpopulate, which may lead to extinctions on the pray of the secondary consumers. ex. A mountain lion would eat rabbits who eat berries, if there are no mountain lions to kill the rabbits, then the berries may be scarce
Explanation:
bc i know everthing
April investigated a crime scene where a gunshot sent blood through the air and it splattered on the wall. How would a forensic scientist BEST categorize this blood stain?
A.
transfer
B.
impact
C.
circular
D.
passive
Answers
A forensic scientist would best investigate categorize the blood stain by b) impact.
It is given that the blood is splattered on the wall. It was splattered by a gunshot and is also given that the blood traveled through air before splattering.
Since, it was a gunshot the trigger from the gun would surely create a force on the Blood which would push the blood to move forward.
This blood when falling onto the surface of the wall would create an impact depending upon the speed it traveled through the air.
Therefore, the crime scene would be best investigated and categorize the blood stain based on impact of the blood shot from the gun and can move to further investigation.
Hence the correct option is b) and the correct answer is impact.
To learn more about crime investigation, click below:
https://brainly.com/question/6203610
#SPJ1
Arrange the following elements in order of decreasing atomic radius: Ba , Sn , Cl , Pb , Se .
Rank elements from largest to smallest.
Answers
Answer:
Ba, Pb, Sn, Se, Cl
Explanation:
The atomic radius increases when going right to left and top to bottom on the periodic table and decreases going the opposite way so I put it in order based on that.
Which of the following is an incorrect representation for a neutral atom?
36Li
613C
3063Cu
1530P
Answers
This representation suggests that the element is phosphorus (P) with a mass number of 30, which is incorrect. The correct mass number for phosphorus is approximately 30.97. The incorrect representation for a neutral atom is 36Li
To determine the correct representation for a neutral atom, we need to consider the atomic number (Z) and mass number (A) of the element. The atomic number represents the number of protons in the nucleus, while the mass number represents the sum of protons and neutrons.
Let's analyze the given representations:
36Li:
This representation suggests that the element is lithium (Li) with a mass number of 36, which is incorrect. The correct mass number for lithium is approximately 6.94.
613C:
This representation suggests that the element is carbon (C) with a mass number of 13, which is correct. Carbon has different isotopes, and 13C represents one of its stable isotopes.
3063Cu:
This representation suggests that the element is copper (Cu) with a mass number of 63, which is correct. Copper has different isotopes, and 63Cu represents one of its stable isotopes.
1530P:
This representation suggests that the element is phosphorus (P) with a mass number of 30, which is incorrect. The correct mass number for phosphorus is approximately 30.97.
Therefore, the incorrect representation for a neutral atom is 36Li, as it does not match the known properties of lithium.
For more question on atom
https://brainly.com/question/26952570
#SPJ8
Express the Following number in scientific notation with 3 significant figures:
450,000
[ ? ] x 10 to the power of ?
Answers
Answer:
4.50x10^5
Explanation:
dont forget the zero on the 4.50
please help me ASAAAAAAAAAAAP
Identify the Arrhenius acid and the Arrhenius base in this reaction.
H2SO4 + 2NaOH → Na2SO4 + 2H2O
Question 35 options:
Na2SO4(acid), 2H2O(base)
H2SO4(acid), NaOH(base)
NaOH(acid), Na2SO4(base)
H2SO4(acid), Na2SO4(base)
Answers
Answer:
H2SO4(acid), NaOH(base)
Explanation:
match the ph range in the left hand column with the most suitable buffer for that ph range in the right hand column.
Answers
The optimal pH for any buffer is when its pKa value coincides with the pH of the solution. A buffer typically functions well throughout a pH range that is 1 unit on either side of the pKa value.
The buffer in the pH range of 6.6 to 8.6 is made up of lactic acid, whose pKa value is 3.86, a PIPES buffer at pH 2.9 to 4.9, an acetic acid buffer at pH 5.8 to 7.8, and a HEPES buffer at pH 3.8 to 5.8. An amalgam of a weak acid and its conjugate base is known as a buffer solution. When basic or acidic components are added, it resists any pH shift. We can divide buffer solutions into two categories. Acidic and basic buffer solutions are the two categories into which we can divide buffer solutions. Because they produce unionised acid or base when they react with acid or base, a buffer solution resists any pH shift.
To learn more about pH click here
https://brainly.com/question/15289741
#SPJ4

What type of reaction is illustrated below?2H1 --> H2 + 12
Answers
Answer:
Decomposition reaction.
Explanation:
2HI —> H₂ + I₂
From the above equation, we can see clearly that HI undergoes a reaction to produce H₂ and I₂ which are the elements that make up HI.
Therefore, the equation illustrated above is a decomposition reaction because HI breaks into H₂ and I₂
NOTE: Decomposition reaction is a reaction in which a compound splits or breakdown into two or more simple elements or compound.
which of the following foams has the capability of re-covering any gaps created by fire fighters walking through and disturbing the foam layer
Answers
Foams have the capability of recovering any gaps created by firefighters walking through and disturbing the foam layer is Aqueous film-forming foam
Fire suppressants like aqueous film-forming foam (AFFF) are used to put out flammable liquid fires like fuel fires.
AFFF is frequently employed in fire fighting apparatus, shipboard and land-based facility fire suppression systems, and fire training facilities. According to the amount of water it is mixed with, AFFF is often sold as a concentrate and is referred to as "3%" or "6%" (Type 3 or Type 6, respectively).
To avoid long-term harmful effects on human health or the environment, containment and cleanup may be necessary when AFFF is used, discharged, or released into the environment.
Learn more about firefighting foam:
https://brainly.com/question/29524190
#SPJ4
When the nuclide bismuth-210 undergoes alpha decay:
The name of the product nuclide is_____.
The symbol for the product nuclide is_____
Fill in the nuclide symbol for the missing particle in the following nuclear equation.
_____ rightarrow 4He+ 234Th
2 90
Write a balanced nuclear equation for the following:
The nuclide radium-226 undergoes alpha emission.
Answers
Explanation:
An atom undergoes alpha decay by losing a helium atom.
So when bismuth undergoes alpha decay, we have;
²¹⁰₈₃Bi --> ⁴₂He + X
Mass number;
210 = 4 + x
x = 206
Atomic number;
83 = 2 + x
x = 81
The element is Thallium. The symbol is Ti.
For the second part;
X --> ⁴₂He + ²³⁴₉₀Th
Mass number;
x = 4 + 234 = 238
Atomic Number;
x = 2 + 90 = 92
The balanced nuclear equation is;
²³⁸₉₂U --> ⁴₂He + ²³⁴₉₀Th
In a chemical process, you need to force a compound to bond with a halogen, causing it to lose hydrogen. Which type of reaction do you need to perform?Question options:A) AmmoniationB) ReductionC) HalogenationD) Hydrolysis
Answers
Explanation:
During the halogenation reaction, there is usually an addition of one or more halogens to the substance. Also, the substance usually loses hydrogren atoms. For example:
CH₄ + Cl₂ ----> CH₃-Cl + HCl
Answer: C) Halogenation.
What element has 12 protons, 12 neutrons and 12 electrons?
Answers
Answer:
magnesium atom
The most common and stable type of magnesium atom found in nature has 12 protons, 12 neutrons, and 12 electrons
A que se denomina función química
Answers
En química, el grupo de algunas sustancias compuestas que poseen propiedades químicas semejantes, denominadas propiedades funcionales, recibe el nombre de función química. ... Además están divididas en ácidos, bases, sales y óxidos; y funciones orgánicas que son las relativas a los compuestos orgánicos.
BRAINLIST
