A car is traveling with an initial velocity of 15 m/s when the driver decides to go faster by accelerating at 5 m/s2 for 10 seconds. How far did the car travel during this time?
70 m
300 m
175 m
400 m
Answers
Answer: Time needed: 2.5 s
Distance covered: 31.3 m
Explanation:
I'll start with the distance covered while decelerating. Since you know that the initial speed of the car is 15.0 m/s, and that its final speed must by 10.0 m/s, you can use the known acceleration to determine the distance covered by
on one side of the equation and solve by plugging your values
To get the time needed to reach this speed, i.e. 10.0 m/s, you can use the following equation
Explanation:
Related Questions
What is the electron configuration for manganese
Answers
Electron configuration for manganese is [Ar]3d⁵4s² and electron configuration is the distribution of electrons of an atom or molecule
The electronic configuration of an element is a symbolic notation of the manner in which the electrons of its atoms are distributed over different atomic orbitals and atomic number of manganese is 25 then electronic configuration [Ar]3d⁵4s² manganese is a chemical element with the symbol Mn and it is a hard, brittle, silvery metal, often found in minerals in combination with iron and atomic mass is 55.93 and we write electronic configuration is 1s²2s²2p⁶3s²3p⁶4s²4d⁵ called as electronic configuration of manganese
Know more about electron configuration
https://brainly.com/question/15489236
#SPJ1
Is alka seltzer and acid or a base or netural
Answers
Answer:
Base
Explanation:
Why is Alka-Seltzer a base?
Alka-Seltzer contains sodium bicarbonate and citric acid. The sodium citrate acts as an antacid. Excess sodium bicarbonate from the Alka-Seltzer acts to neutralize base.
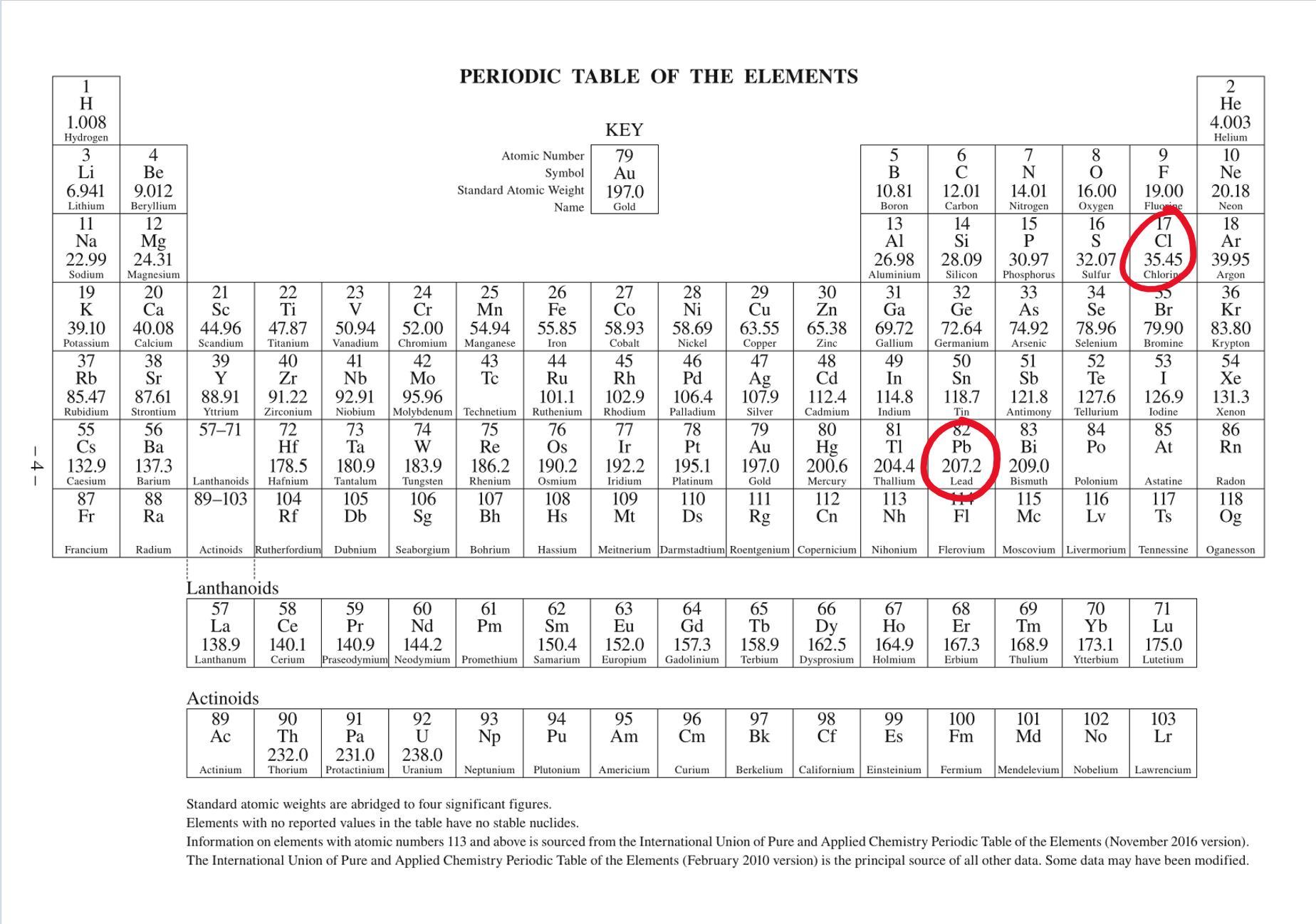
What’s the molar mass of lead(II) chloride
Answers
Answer:
molar mass = 278.1 g/mol
Explanation:
Molar mass is the mass of one mole of element, compound, molecule, etc.
It has the units of grams per mole (g/mol), and in mole calculations, is commonly denote the symbol M.
Lead(II) chloride has the chemical formula:
\(\boxed{\rm PbCl_2}\)
The molar mass of lead chloride, is the total sum of all the individual molar masses of each element. See attached image, standard IUPAC Periodic Table found in data sheets/booklets for most chemistry exams around the world.
Hence molar mass = (207.2)+(35.45)×2 = 278.1 g/mol
To learn more about molar mass:
https://brainly.com/question/30640134
If a jet traces 395,000 meters in 9000 seconds. What was the speed of the jet?
Answers
Answer:
43.89 m/sExplanation:
The speed of the jet can be found by using the formula
\(v = \frac{d}{t} \\ \)
d is the distance
t is the time taken
From the question we have
\(v = \frac{395000}{9000} = \frac{395}{9} \\ = 43.8888...\)
We have the final answer as
43.89 m/sHope this helps you
Sodium phosphate reacts with sulfuric acid to form sodium sulfate and phosphoric acid. What is the stoichiometric coefficient for sulfuric acid when the chemical equation is balanced using the lowest whole-number stoichiometric coefficients?.
Answers
When sodium phosphate reacts with sulfuric acid, forming sodium sulfate and phosphoric acid, the stoichiometric coefficient for sulfuric acid in the balanced chemical equation is 3.
In every balanced chemical equation, the total number of individual atoms on the reactant side must be equal to the total number of individual atoms on the product side. The stoichiometric coefficient is the number written in front of each reactants and products that tells how many moles of each are needed in the reaction.
The chemical equation for the given reaction is:
\(Na_{3} PO_{4}\) + \(H_{2} SO_{4}\) = \(Na_{2} SO_{4}\) + \(H_{3} PO_{4}\)
Put the necessary stoichiometric coefficient to balance each element.
Balancing Na:
\(2Na_{3} PO_{4}\) + \(H_{2} SO_{4}\) = \(3Na_{2} SO_{4}\) + \(H_{3} PO_{4}\)
Balancing P:
\(2Na_{3} PO_{4}\) + \(H_{2} SO_{4}\) = \(3Na_{2} SO_{4}\) + \(2H_{3} PO_{4}\)
Balancing S:
\(2Na_{3} PO_{4}\) + \(3H_{2} SO_{4}\) = \(3Na_{2} SO_{4}\) + \(2H_{3} PO_{4}\)
Notice that H and O are already balanced.
Hence, the balanced chemical equation for the reaction is:
\(2Na_{3} PO_{4}\) + \(3H_{2} SO_{4}\) = \(3Na_{2} SO_{4}\) + \(2H_{3} PO_{4}\)
where 3 is the stoichiometric coefficient of sulfuric acid, \(H_{2} SO_{4}\).
Learn more about stoichiometric coefficient here: https://brainly.com/question/6666875
#SPJ4
15)A photon has a frequency of 2.68 x 106 Hz. Calculate its energy.
Can you please show how you got it?
Answers
To solve that, you can use Planck’s equation, ok?
E = hf
E = [6.63*10^(-34)]*[2.68*10^(6)]
E = 17,77*10^(-28) = 1,77*10^(-27) J
So its energy is 1,77*10^(-27) J.
A photon has a frequency of 2.68 × \(10^6\) Hz then the energy of a photon is \(1.78\times10^{-27}\text{J}\).
What is meant by Planck's equation?
Planck's energy–frequency relation, or Planck's equation, gives the relationship between the energy of a photon and frequency. This equation is given by,
E = hv
Where, E be the energy of a photon
h be the Planck constant
v be the frequency
Given:
Frequency is 2.68 × \(10^6\)
Planck's constant is 6.63 × \(10^{-34}\) Js
The energy of a photon is given by,
E = hv
substitute the values in the above equation, we get
= 6.63 × \(10^{-34}\) Js × 2.68 × \(10^6\) Hz
simplifying the above equation, we get
= 1.78 × \(10^{-27}\) J
The energy of the photon is 1.78 × \(10^{-27}\) J.
To learn more about photon refer to:
https://brainly.com/question/15946945
#SPJ2
consider the reaction: kclo4(s) kcl(s) 2o2(g) and the table of values given on the right. do you expect this reaction to be spontaneous at room temperature? why? g
Answers
Based on the table of values given on the right, the reaction of KCLO4(s) to KCl(s) and 2O2(g) is expected to be spontaneous at room temperature.
The spontaneity of a reaction can be determined by its Gibbs free energy change (ΔG). If ΔG is negative, the reaction is spontaneous and can occur without external energy input. If ΔG is positive, the reaction is non-spontaneous and requires external energy input. The equation for calculating ΔG is: ΔG = ΔH - TΔS, where ΔH is the enthalpy change, T is the temperature in Kelvin, and ΔS is the entropy change.
The table of values given on the right shows that the enthalpy change (ΔH) for the reaction is -390.2 kJ/mol, which is exothermic. The entropy change (ΔS) for the reaction is also positive, indicating an increase in disorder in the system. Therefore, plugging in the given values into the equation ΔG = ΔH - TΔS, we get:
ΔG = -390.2 kJ/mol - (298 K) * (0.2202 kJ/K*mol)
ΔG = -390.2 kJ/mol - 65.64 kJ/mol
ΔG = -455.84 kJ/mol
Since ΔG is negative, this means that the reaction is spontaneous and can occur without external energy input.
Therefore, based on the table of values given on the right, we can expect the reaction of KCLO4(s) to KCl(s) and 2O2(g) to be spontaneous at room temperature.
To know more about temperature, visit;
https://brainly.com/question/26866637
#SPJ11
How does the number of protons affect the atom?
Answers
Protons are the subatomic particles which has positive charge.
The protons define the element.
So for example, every element that has 1 proton is hydrogen.
Hydrogen is a gas.
Every element that has 23 protons would be vanadium.
Vanadium is a chemical element with an atomic number 23.
true or false: sn2 mechanism for nucleophilic substitution reactions involves one step and occurs with inversion of configuration.
Answers
Sₙ2 mechanism for nucleophilic substitution reactions involves one step and occurs with inversion of configuration. - True
The rate determining step in Nucleophilic bimolecular Substitution reaction involves the presence of both substrate and the attacking nucleophile as in this mechanism the the process of bonding of substrate with the nucleophile takes place simultaneously with the process in which the leaving group is leaving the substrate. \
Rate α [Substrate][attacking nucleophile]
So, the reaction takes place in one step.
Also, the attacking nucleophile attacks on the opposite side of the leaving group of the substrate, so this reaction occurs with inversion in configuration.
To know more about nucleophilic substitution reactions here
https://brainly.com/question/28097754
#SPJ4

what is the percent composition of salicylic acid?
Answers
The percent composition of salicylic acid is C7H6O3, or 60.87%C, 4.4%H, and 34.75%O
Explain why for static electricity to occur between two surfaces that are rubbed against each other, one of the materials has to be an insulator
Answers
When two surface come in contact and rub each other a frictional force causing them s deformation and this leads to a voltage giving rise to a static charge.
What is static charge?Static charge is developed within a body temporarily with an induced polarization. These charges are not moving and set into a pole in the material.
When two materials rub against each other in which one is a conductor and other to be an insulator, they will attract by the induced polarization. Rubbing causes the free electrons in the condutor to be transferred towards the insulator.
This will cause a deformation that, the random charges in the material get polarized where the electrons in the insulator will repel to the opposite pol and positive charges will align in a pole close to the condutor.
This charge separation causes the positive pole of the insulator gets attracted into the conductor surface. Hence, there forms a static electricity by the passage of electron.
To find more on static electricity, refer here:
https://brainly.com/question/12791045
#SPJ5
El agua oxigenada es una disolucion al 7% en volumen de peroxido de hidrogeno en agua , que se usa para destruir microorganismos en heridas leves y asi de reducir la posibilidad de infeccion en los tejidos. El volumen de peroxido de hidrogeno nesesario para preparar un litro en disolucion al 6% en volumen es:
Answers
Answer:
857mL de la solución al 7%
Explanation:
Una solución de agua oxigenada (H₂O₂) al 7% contiene 7mL de peróxido de hidrógeno por cada 100mL de solución = 7mL H₂O₂ / 100mL Sln
Para preparar un litro = 1000mL de disolución al 6% de peróxido de hidrógeno se requieren:
1000mL Sln * (6mL H₂O₂ / 100mL Sln) = 60mL H₂O₂
Para obtener 60mL de H₂O₂ a partir de la solución al 6% se requieren:
60mL H₂O₂ * (100mL Sln / 7mL H₂O₂) =
857mL de la solución al 7%Calculate the volume, in milliliters, of a 0.750 m koh solution that should be added to 5.250 g of hepes (mw = 238.306 g/mol, pa = 7.56 ) to give a ph of 7.64.
Answers
Based on the given information, it is not possible to determine the volume of the 0.750 M KOH solution needed or the pH of the HEPES solution.
To calculate the volume of a 0.750 M KOH solution that needs to be added to 5.250 g of HEPES to achieve a pH of 7.64, we need to follow a step-by-step process.
1. Determine the moles of HEPES:
First, calculate the number of moles of HEPES using its molecular weight (MW = 238.306 g/mol). Divide the mass of HEPES (5.250 g) by its molecular weight:
Moles of HEPES = 5.250 g / 238.306 g/mol
2. Determine the volume of KOH solution required:
To neutralize the acidic HEPES solution, we need to use the HEPES to KOH ratio based on the balanced equation. However, the balanced equation is not provided in the question. Therefore, it is not possible to determine the exact volume of KOH solution required.
3. Calculate the pH of the HEPES solution:
The question states that the desired pH is 7.64. However, the pKa (acid dissociation constant) of HEPES is given (pKa = 7.56). This indicates that HEPES acts as a buffer, and its pH can be controlled within a specific range. However, without the buffer capacity or the concentration of HEPES solution, we cannot accurately determine the pH.
Additional information such as the buffer capacity or the concentration of the HEPES solution is required.
Learn more about solution here:-
https://brainly.com/question/1616939
#SPJ11
What compound do plants use to absorb the energy from sunlight?.
Answers
Plants use a compound called chlorophyll to absorb the energy from sunlight.
Chlorophyll is a pigment found in the chloroplasts of plant cells, particularly in the leaves. It plays a crucial role in the process of photosynthesis, which is how plants convert sunlight into chemical energy.
Chlorophyll absorbs light in the visible spectrum, particularly in the red and blue regions, while reflecting green light, which is why plants appear green to our eyes. When chlorophyll molecules absorb photons of light, they become energized. This energy is then used to power the process of photosynthesis, where carbon dioxide and water are converted into glucose (a form of sugar) and oxygen.
However, chlorophyll is the compound in plants that captures and harnesses the energy from sunlight, initiating the process of photosynthesis and allowing plants to produce the energy-rich molecules they need for growth and survival.
To know more about Chlorophyll here
https://brainly.com/question/15867555
#SPJ4
what is the emperical formular for C6H6
Answers
Answer:
It’s a steppingstone in finding the molecular formula of an unknown compound.
Explanation:
Benzene has empirical formula of CH
Ethylene Glycol is an organic compound.
It has a molecular formula of C6H6
Which means it has 6 carbon atoms, and 6 hydrogen atoms.
To find the empirical formula for any compound we need to know how many atoms of different types are present like in case of benzene there were 6 carbon ,and 6 hydrogen atoms
empirical formula means we need to get the simplest ratio of all these atoms, so here we would divide the each different number of atoms by 6 as all these have 6 as a common factor
6 / 6 = 1 ( for Carbon )
6 / 6 = 1 ( for Hydrogen )
Hence the empirical formula becomes : C1H1 / CH
estimate the freezing point of 1 liter of water to which a) 25 g of glucose have been added; b) 25 g of sucrose have been added; and, c) 25 g of sodium chloride have been added.
Answers
a) Adding 25 g of glucose depresses the freezing point of water by approximately 0.26 °C. b) Adding 25 g of sucrose depresses the freezing point of water by approximately 0.14 °C. c) Adding 25 g of sodium chloride depresses the freezing point of water by approximately 0.80 °C.
The freezing point of a solution is lower than the freezing point of pure water due to the presence of solute particles. The extent of this depression depends on the concentration and nature of the solute.
To estimate the freezing point depression, we can use the formula:
ΔT = Kf * m
Where:
ΔT = freezing point depression
Kf = cryoscopic constant (a property of the solvent)
m = molality of the solution (moles of solute per kilogram of solvent)
For water, the cryoscopic constant (Kf) is approximately 1.86 °C/m.
Now let's calculate the molality (m) of each solution:
a) Glucose (C6H12O6)
The molar mass of glucose is 180.16 g/mol.
Molality (m) = moles of solute / mass of solvent (in kg)
= (25 g / 180.16 g/mol) / 1 kg
= 0.1386 mol/kg
ΔT_a = Kf * m_a
ΔT_a = 1.86 °C/m * 0.1386 mol/kg
ΔT_a ≈ 0.2579 °C
Therefore, the estimated freezing point of 1 liter of water with 25 g of glucose added is approximately -0.26 °C.
b) Sucrose (C12H22O11)
The molar mass of sucrose is 342.30 g/mol.
Molality (m) = moles of solute / mass of solvent (in kg)
= (25 g / 342.30 g/mol) / 1 kg
= 0.0729 mol/kg
ΔT_b = Kf * m_b
ΔT_b = 1.86 °C/m * 0.0729 mol/kg
ΔT_b ≈ 0.1355 °C
Therefore, the estimated freezing point of 1 liter of water with 25 g of sucrose added is approximately -0.14 °C.
c) Sodium Chloride (NaCl)
The molar mass of sodium chloride is 58.44 g/mol.
Molality (m) = moles of solute / mass of solvent (in kg)
= (25 g / 58.44 g/mol) / 1 kg
= 0.4279 mol/kg
ΔT_c = Kf * m_c
ΔT_c = 1.86 °C/m * 0.4279 mol/kg
ΔT_c ≈ 0.7954 °C
Therefore, the estimated freezing point of 1 liter of water with 25 g of sodium chloride added is approximately -0.80 °C.
Therefore,
a) Adding 25 g of glucose depresses the freezing point of water by approximately 0.26 °C.
b) Adding 25 g of sucrose depresses the freezing point of water by approximately 0.14 °C.
c) Adding 25 g of sodium chloride depresses the freezing point of water by approximately 0.80 °C.
To know more about freezing point visit:
https://brainly.com/question/40140
#SPJ11
Calculate the number of formula units in 12. 5 mol of calcium carbonate CaCO3
Answers
12.5 mol of the substance contains 7.53 x 10²⁴ formula units of calcium carbonate.
Calcium carbonate (CaCO₃) is a compound that consists of one calcium atom, one carbon atom, and three oxygen atoms. The formula unit of calcium carbonate contains one Ca atom, one C atom, and three O atoms. To calculate the number of formula units in 12.5 mol of calcium carbonate, we need to use Avogadro's number, which relates the number of particles (atoms, molecules, or formula units) in a given amount of substance.
Avogadro's number (Nₐ) is 6.022 x 10²³ particles/mol. Therefore, the number of formula units of calcium carbonate in 12.5 mol can be calculated as:
n = Nₐ * 12.5 mol
n = 6.022 x 10²³ particles/mol * 12.5 mol
n = 7.53 x 10²⁴ particles
Therefore, there are 7.53 x 10⁴ formula units of calcium carbonate in 12.5 mol of the compound.
To learn more about calcium carbonate refer to:
brainly.com/question/13565765
#SPJ4
step 2: let's consider the mechanism to convert a carboxylic acid to an ester. which step happens first? the carbonyl oxygen is protonated. the hydroxy oxygen is protonated. the alcohol adds to the carbonyl to form a tetrahedral intermediate. the alcohol displaces the hydroxy group in an sn2 reaction.
Answers
The correct order of the steps is: carbonyl oxygen is protonated, alcohol adds to the carbonyl to form a tetrahedral intermediate, hydroxy oxygen is protonated, and alcohol displaces the hydroxy group in an SN2 reaction.
The first step in the mechanism to convert a carboxylic acid to an ester is the protonation of the carbonyl oxygen. This protonation makes the carbonyl carbon more electrophilic, allowing it to react with the alcohol in the next step. The alcohol then adds to the carbonyl to form a tetrahedral intermediate. The hydroxy oxygen is then protonated, which facilitates the leaving of the hydroxy group and the formation of the ester in an SN2 reaction.
Tetrahydrofuran (THF) and hydrogen sulphide (HSCH3) react in the specified reaction using an SN2 mechanism. SN is a particular kind of nucleophilic substitution reaction in which the nucleophile contacts the substrate directly, which causes the leaving group to be displaced in a bimolecular manner. As the nucleophile in the described reaction, hydrogen sulphide attacks the THF's carbon center. Through a coordinated process, this assault causes the leaving group—an alkyl group linked to the THF—to be displaced.
Learn more about SN2 reaction here
https://brainly.com/question/32099348
#SPJ11
Which properties change the composition of a substance? A physical properties
B chemical properties C chemical and physical properties D neither chemical nor physical properties
Answers
Answer:
chemical properties
Explanation:
chemical properties did change the composition of substance, so it cannot go back to original anymore, such as burning, rusting, etc.
I took the test before. I got all the answer correct and got 100% score.
You can see my other article just search in search engine with: Learningandassignments diy4pro
Click on my site and find these related article post:
Learning Properties of Matter Quiz Unit 2 Lesson 11 Grade 8
Hope it helps.
#diy4pro
for a particular redox reaction, no−2 is oxidized to no−3 and cu2 is reduced to cu . complete and balance the equation for this reaction in basic solution. phases are optional.
Answers
Therefore, the balanced equation for the redox reaction in basic solution is:
2NO2- + Cu2+ + 4OH- → 2NO3- + Cu + 2H2O
The balanced equation for the redox reaction in basic solution is:
2NO2- + Cu2+ + 4OH- → 2NO3- + Cu + 2H2O
In this reaction, NO2- is oxidized (loses electrons) to NO3- and Cu2+ is reduced (gains electrons) to Cu. The reaction takes place in basic solution, which means that we need to balance the equation by adding OH- ions to balance out the H+ ions.
To balance the equation, we first balance the atoms in each half-reaction:
Oxidation half-reaction:
NO2- → NO3-
Add 2H2O and 4e- to the left side to balance the charge and atoms:
NO2- + 2H2O + 4e- → NO3-
Reduction half-reaction:
Cu2+ → Cu
Add 2e- to the left side to balance the charge:
Cu2+ + 2e- → Cu
Next, we balance the number of electrons transferred by multiplying each half-reaction by the appropriate factor:
Multiply oxidation half-reaction by 2:
2NO2- + 4H2O + 8e- → 2NO3-
Multiply reduction half-reaction by 4:
4Cu2+ + 8e- → 4Cu
Now we add the two half-reactions together, canceling out the electrons on both sides:
2NO2- + 4H2O + 8e- + 4Cu2+ → 2NO3- + 4Cu + 8OH-
Finally, we simplify the equation by canceling out the H2O molecules and reducing the coefficients:
2NO2- + 4Cu2+ + 4OH- → 2NO3- + 4Cu + 2H2O
To know more about balanced equation visit:
https://brainly.com/question/7181548
#SPJ11
An atom has a mass number of 9 and 5 neutrons What is its atomic number?
A. 14
B. 11
C. 19
D. 4
Answers
Answer:
D. 4
Explanation:
mass number - number of neutrons = number of protons
If there is something floating in a liquid, what is the best way to separate the two chemicals?
-distillation
-evaporation
-filtration
-sorting
Answers
Answer:
Filtration
explanation:
Filtration is a separation method used to separate out pure substances in mixtures comprised of particles—some of which are large enough in size to be captured with a porous material. Particle size can vary considerably, given the type of mixture. For instance, stream water is a mixture that contains naturally occurring biological organisms like bacteria, viruses, and protozoa. Some water filters can filter out bacteria, the length of which are on the order of 1 micron. Other mixtures, like soil, have relatively large particle sizes, which can be filtered through something like a coffee filter.
How many atoms are in 2 moles of Ca
Answers
Mole is a unit of measurement used to measure the amount of a chemical substance, with 1 mole containing 6.022 x 1023 atoms, or 1.204 x 1024 atoms.
What is a mole?A mole is a unit of measurement in chemistry that is used to determine the quantity of a chemical. It is described as the quantity of a material containing the same number of particles (atoms, molecules, ions, and so on) as there are atoms in 12 grammes of carbon-12.
2 moles of Ca have 2 x 6.022 x 1023 atoms. This is due to the fact that the mole (mol) is a unit of measurement used to determine the quantity of a chemical compound. 1 mol of a material corresponds to 6.022 x 1023 atoms of that substance. As a result, 2 moles of Ca contain 2 x 6.022 x 1023 atoms, or 1.204 x 1024 atoms.
To know more about mole, visit
brainly.com/question/26416088
#SPJ1
2. Low-density polyethylene, used to make plastic films, is
made by the radical polymerization of ethene, and is one of
the hardest plastics to recycle. It gets its name
A. from the process by which it's polymerized.
B. because of the way the chains pack like spaghetti, close
and tight.
C. from the spaces left between chains when the polymer is
formed.
D. because it's strong but flexible.
Answers
Answer:
from the spaces left between chains when the polymer is
formed.
Explanation:
Polyethylene polymer may be of high or low density. Whether the polymer is of high or low density depends on the arrangement of the polymer chains.
If the polymer chains are close together such that the resultant polymer is crystalline with chains packed closely, we have high density polyethylene.
On the other hand if the chains are not close together and there are spaces left between chains when the polymer is formed, then we have low density polyethylene.
Hence, low density polyethylene gets its name from the spaces left between chains when the polymer is formed.
Answer: it gets its name (from the process by which it's polymerized). Therefore the correct option is A.
Explanation:
Polyethylene is a type of plastic which is made by the polymerization of its monomers (ethene). The most common monomers are derived from petrochemicals. The monomers simply join together to form the polymers which has the same empirical formula as the monomer, but is of a higher molecular mass.
There are two main types of polyethylene, these include:
--> Low density polyethylene: when ethene is subject to a high temperature of about 250°C and pressure above 1500atm, together with traces of oxygen ( as an initiator), it polymerizes to polyethylene. The polyethylene obtained by the above method is called a low density polyethylene. It has the most long- and short-chain branching of any form of polyethylene, resulting in its lower density. Therefore its name is gotten from the process by which it's polymerized
--> High density polyethylene: this is prepared at a lower temperature and pressure by using catalysts.
FILL IN THE BLANK
Valence Electrons: Nonmetals v.s. Metals
Valence electrons in nonmetals occupy directional ___-orbitals.
Hardest substances known
Brittle, given enough ___
Metal valence electrons spread out into ___ s-orbitals.
Bonds do not "shatter"
Easily deformed (___)

Answers
Valence electrons in non-metal occupy directional p-orbitals.
Hardest substances known.Brittle, given enough force.Metal valence electrons spread out into spherical s-orbitals.
Bonds do not "shatter"Easily deformed (malleable).What are valence electrons?Valence electrons can be defined as the number of electrons that are present in the outermost shell of an atom. Also, valence electrons are typically used to determine whether an atom or group of elements found in a periodic table can bond with others.
This ultimately implies that, valence electrons is a property is typically used to determine the chemical properties of chemical elements.
What is a sublevel?A sublevel can be defined as an energy level that is associated with the electrons found outside the atomic nucleus.
The types of sublevel.In Chemistry, there are four (4) types of sublevel and these include the following:
I. s-orbital (sublevel): it has one (1) orbital i.e 1s.
II. p-orbital (sublevel): it has three (3) orbitals.
III. d-orbital (sublevel): it has five (5) orbitals.
IV. f-orbital (sublevel): it has seven (7) orbitals.
Read more on orbitals and electrons here: https://brainly.com/question/11404939
#SPJ1
How many electrons in a water molecule?
Answers
The water molecule has a total of 10 protons and 10 electrons (8 from the oxygen atom and 1 from each of the two hydrogen atoms). Since it has the same number of protons and electrons, the water molecule is neutral. The electron cloud model shows where electrons are in a molecule.
- BRAINLIEST answerer
during the light reactions, water is oxidized to o2. where do the hydrogens go?
Answers
The hydrogen will mix with NADP+ to create NADPH, which will then be sent to the dark cycle.
What is the role of hydrogen in light reactions?The oxygen molecule is created when the elemental oxygen from one water molecule joins with oxygen from another water molecule.
The hydrogen will mix with NADP+ to create NADPH, which will then be sent to the dark cycle. They are the source of oxygen, as explained. Chlorophyll, a pigment found inside photosystem II of the chloroplast, is where this occurs. The Mg ion in this chlorophyll pigment experiences redox state variations (after interaction with photon). In the process, hydrogen ions are removed from the water molecule, which leads to the oxidation of the water molecule to O2. While this is happening, the hydrogen is expelled from the thylakoid and interacts with NADP+ to create NADPH (reduction process). This NADPH travels to the calvin cycle. The Rubisco enzymes are also found in the stroma area. The next enzyme, glyceraldehyde 3-phosphate dehydrogenase, is outside of the chloroplast and utilises NADPH itself. Because rubisco does not require photons of light to work, the dark cycle is the component that involves rubisco.
To know more about hydrogen, visit:
https://brainly.com/question/28937951
#SPJ1
Predict the products of the following reaction: Zn(ClO₃)₂ (aq) + K₃PO₄ (aq)
Answers
The products : Zn₃(PO₄)₂ (s) + KClO₃ (aq)
Further explanationGiven
reaction: Zn(ClO₃)₂ (aq) + K₃PO₄ (aq)
Required
The products
Solution
Double-Replacement reactions : an ion exchange between two ion compounds in the reactant to form two new ion compounds in the product
General formula :
AB + CD ⇒ AD + CB
One of the characteristics of the double replacement reaction is the presence of precipitated compounds
Zn(ClO₃)₂ (aq) + K₃PO₄ (aq) ⇒ Zn₃(PO₄)₂ (s) + KClO₃ (aq)
Zn₃(PO₄)₂ (s)⇒ precipitated compounds, so that this reaction can occur
A mixture of three noble gases has a total pressure of 1.25 atm. The individual pressures exerted by neon and argon are 0.22 atm and 0.35 atm, respectively. What is the partial pressure of the third gas, helium?
Group of answer choices
0.57 atm
0.68 atm
1.38 atm
1.82 atm
Answers
which component of the sample, ethyl acetate or ethyl butyrate, elutes faster?
Answers
Ethyl acetate has a lower boiling point than ethyl butyrate, and it is also more polar, so it is less strongly adsorbed to the stationary phase and elutes faster. Therefore, ethyl acetate is the component of the sample that elutes faster than ethyl butyrate. The answer to the question is ethyl acetate.
Ethyl acetate elutes faster than ethyl butyrate. Gas chromatography is a method used to separate and analyze mixtures of compounds, and it is often used in the flavor and fragrance industry. Gas chromatography separates mixtures of compounds by passing them through a column with a stationary phase, where each component is adsorbed to varying degrees, and then the components are eluted from the column and detected.
Ethyl acetate and ethyl butyrate are two compounds that are commonly analyzed by gas chromatography. The compound that elutes faster is the one that is less strongly adsorbed to the stationary phase. The boiling point and polarity of the compound determine its strength of adsorption. Ethyl acetate has a lower boiling point than ethyl butyrate, and it is also more polar, so it is less strongly adsorbed to the stationary phase and elutes faster. Therefore, ethyl acetate is the component of the sample that elutes faster than ethyl butyrate. The answer to the question is ethyl acetate.
Learn more About Ethyl acetate from the given link
https://brainly.com/question/28169580
#SPJ11