Answers
Answer:
6. 13.8 moles
7. 0.751 moles
8. 2.04 moles
9. 0.00794 moles
10. 0.158 moles
Explanation:
Answer:
6. 13.8 moles
7. 0.751 moles
8. 2.04 moles
9. 0.00794 moles
10. 0.158 moles
Explanation:
Related Questions
When sodium (Na) is mixed with water, an explosion takes place. Based on its location on the periodic table, how will potassium (K) behave with water?PLS HELP ME
a) Potassium is in the same period as sodium and shares similar properties so it will explode.
b) Potassium has the same number of valence electrons as sodium so it will explode in water.
c). Potassium is a non metal but sodium is a metal so potassium will not explode.
d) Potassium is in the same group as sodium so it does not share similar chemical properties and it will not explode.

Answers
Answer:d
Explanation:
A sample of Nitrogen gas (N) at 17° C is in a 575 mL container under 85,000 mmHG of pressure. What is the mass of the sample, in grams?
Answers
Answer:
Mass = 75.6 g
Explanation:
Given data:
Temperature of gas = 17°C
Volume of gas = 575 mL
Pressure of gas = 85000 mmHg
Mass of gas = ?
Solution:
Temperature of gas = 17°C (17+273 =290 K)
Volume of gas = 575 mL (575/1000 = 0.575 L)
Pressure of gas = 85000 mmHg (85000/760 = 111.8 atm)
Formula:
PV = nRT
P= Pressure
V = volume
n = number of moles
R = general gas constant = 0.0821 atm.L/ mol.K
T = temperature in kelvin
111.8 atm × 0.575 L = n×0.0821 atm.L/ mol.K × 290 K
64.285 atm. L = n×23.809 atm.L/ mol
n = 64.285 atm. L / 23.809 atm.L/ mol
n = 2.7 mol
Mass of nitrogen gas:
Mass = number of moles × molar mass
Mass = 2.7 mol × 28 g/mol
Mass = 75.6 g
A 175.0 mL solution containing 0.1331 g of a monobasic amine is titrated coulometrically using hydrogen ions generated by the oxidation of water. 2H
2
O(1)⇌4H
+
(aq)+O
2
( g)+4e
−
The end point of the titration occurred at a time of 1095.2 s when a current of 145.0 mA was used. Determine the molar mass of the amine. molar mass y/mol
Answers
The molar mass of the amine will be approximately 325.1 g/mol.
To determine the molar mass of the amine, we need to use the information provided about the titration and the Faraday's laws of electrolysis.
Given;
Volume of solution (V) = 175.0 mL = 0.1750 L
Mass of amine (m) = 0.1331 g
Time of titration (t) = 1095.2 s
Current (I) = 145.0 mA = 0.1450 A
We can start by calculating the number of moles of electrons (n) transferred during the titration using Faraday's law;
n = (Q) / (F)
where Q is the total charge passed (Q = I × t) and F is the Faraday constant (F = 96485 C/mol).
Q = (0.1450 A) × (1095.2 s)
Q ≈ 158.424 C
n = 158.424 C / 96485 C/mol
n ≈ 0.00164 mol
Since the amine is monobasic, it will react with one mole of H⁺ ions (H₃O⁺) per mole of amine.
From the balanced equation;
1 mole of amine reacts with 4 moles of electrons
So, the moles of amine (n_amine) can be calculated using the relationship:
n_amine = n / 4
n_amine ≈ 0.00164 mol / 4
n_amine ≈ 0.000410 mol
Now, we calculate the molar mass (M) of the amine using the formula;
M = m / n_amine
where m is the mass of the amine.
M = 0.1331 g / 0.000410 mol
M ≈ 325.1 g/mol
Therefore, the molar mass of the amine is 325.1 g/mol.
To know more about molar mass here
https://brainly.com/question/31545539
#SPJ4
Explain how understanding this relaship can help desighn more efficient technologist
Answers
Metals are good conductor of electricity, because as per there structure they have free electrons which help them to move freely within the metal thus helps to maintain a good flow of electricity.
This theory has really helped engineers especially for making powerful batteries. As good batteries means amount of electricity drawn from it will be great and that too for longer time.
Hence such metals are used to make batteries that has more number of free electrons and thus can contribute to generate more electricity.
Help with theses two different problems!
1.) 125mL of what is added to 45.3mL of 0.71m NaOH solution
2.) 550mL of water is added to 125mL of 3.01M KOH solution
Answers
1. the final concentration of NaOH after adding 125 mL of water to 45.3 mL of 0.71 M NaOH solution is approximately 0.189 M.
2. the final concentration of KOH after adding 550 mL of water to 125 mL of 3.01 M KOH solution is approximately 0.557 M.
1.) If 125 mL of water is added to 45.3 mL of a 0.71 M NaOH solution, the resulting solution will be a diluted NaOH solution. The addition of water will increase the total volume while reducing the concentration of NaOH. To determine the final concentration of NaOH, we need to consider the conservation of moles.
First, let's calculate the moles of NaOH in the initial solution:
moles of NaOH = volume (in L) × concentration (in M)
moles of NaOH = 0.0453 L × 0.71 M = 0.0321433 moles
After adding 125 mL (0.125 L) of water, the total volume of the solution becomes 0.0453 L + 0.125 L = 0.1703 L.
To find the final concentration, we divide the moles of NaOH by the total volume:
final concentration of NaOH = moles of NaOH / total volume
final concentration of NaOH = 0.0321433 moles / 0.1703 L ≈ 0.189 M
Therefore, the final concentration of NaOH after adding 125 mL of water to 45.3 mL of 0.71 M NaOH solution is approximately 0.189 M.
2.) If 550 mL of water is added to 125 mL of a 3.01 M KOH solution, the resulting solution will also be a diluted solution. Again, we will apply the conservation of moles to determine the final concentration of KOH.
First, calculate the moles of KOH in the initial solution:
moles of KOH = volume (in L) × concentration (in M)
moles of KOH = 0.125 L × 3.01 M = 0.37625 moles
After adding 550 mL (0.55 L) of water, the total volume of the solution becomes 0.125 L + 0.55 L = 0.675 L.
To find the final concentration, divide the moles of KOH by the total volume:
final concentration of KOH = moles of KOH / total volume
final concentration of KOH = 0.37625 moles / 0.675 L ≈ 0.557 M
For more such questions on concentration visit:
https://brainly.com/question/28564792
#SPJ8
Amount of benzil used in grams (g) 0. 147 Amount of sodium borohydride used in grams (g) 0. 021
Product obtained in grams 0. 144 Product melting point (°C) 132-134 Melting point for benzoin mixture (°C) 135-137 Melting point for meso-hydrobenzoin mixture (°C) 127-132 Calculations and Analysis - Amount of reactant in moles ____
- Amount of sodium borohydride in moles. ____ - Product obtained in moles _____ - Product theoretical yield ____
- Product percent yield ____
Answers
Amount of reactant in moles is 0.000693 mol.
Amount of sodium borohydride in moles is 0.000556 mol.
Product obtained in moles is 0.000693 mol.
Product theoretical yield is 0.000693 mol.
Product percent yield is 59.1%.
We can use the given masses of benzil and sodium borohydride to calculate the amount of each reactant in moles. Then, we can use the stoichiometry of the reaction to determine the theoretical yield of the product in moles.
Finally, we can calculate the percent yield of the product using the actual yield (given as 0.144 g) and the theoretical yield.
The balanced chemical equation for the reaction is:
\(C_14H_{10}O_2 + 4 H_3BCN - C_14H_12O_2 + 4 B(OH)^3 + 2 H_2\)
From the molecular formula of benzil \((C_{14}H_{10}O_2)\), we can calculate its molar mass:
Molar mass of benzil = 2(12.01 g/mol) + 10(1.01 g/mol) + 2(16.00 g/mol)
= 212.24 g/mol
Amount of benzil in moles = 0.147 g / 212.24 g/mol
= 0.000693 mol
From the molecular formula of sodium borohydride \((NaBH_4)\), we can calculate its molar mass:
Molar mass of \(NaBH_4\) = 22.99 g/mol + 4(1.01 g/mol) + 4(1.01 g/mol)
= 37.83 g/mol
Amount of sodium borohydride in moles = 0.021 g / 37.83 g/mol
= 0.000556 mol
According to the balanced chemical equation, the reaction produces one mole of product (benzoin) for every one mole of benzil reacted. Therefore, the theoretical yield of benzoin in moles is equal to the amount of benzil used in moles:
Product obtained in moles = 0.000693 mol
Product theoretical yield = 0.000693 mol
Product percent yield = (actual yield / theoretical yield) x 100%
Product percent yield = (0.144 g / (0.000693 mol x 212.24 g/mol)) x 100%
≈ 59.1%
The melting point of the product (132-134°C) falls within the expected range for benzoin, so the product is likely pure.
Learn more about Sodium borohydride at
brainly.com/question/31321350
#SPJ4
Use dimensional analysis to convert 74.9 dry quarts to liquid quarts.
Answers
Answer:
87.2 liquid quarts (US)
Explain how the two atoms in a chlorine molecule are held together
Answers
The chlorine atoms can be held together by the dispersion forces.
How are the chlorine atoms held together?We know that the chlorine atoms are the atoms that we could say that they have an electronegativity value that is almost the same. As such we can see that the the compound is non polar.
Given the fact that the compound is non polar, the atoms would be held together by a kind of bond that not polar in nature and these are the dispersion forces that hold the non polar molecules together.
Learn more about dispersion forces:https://brainly.com/question/14958417
#SPJ1
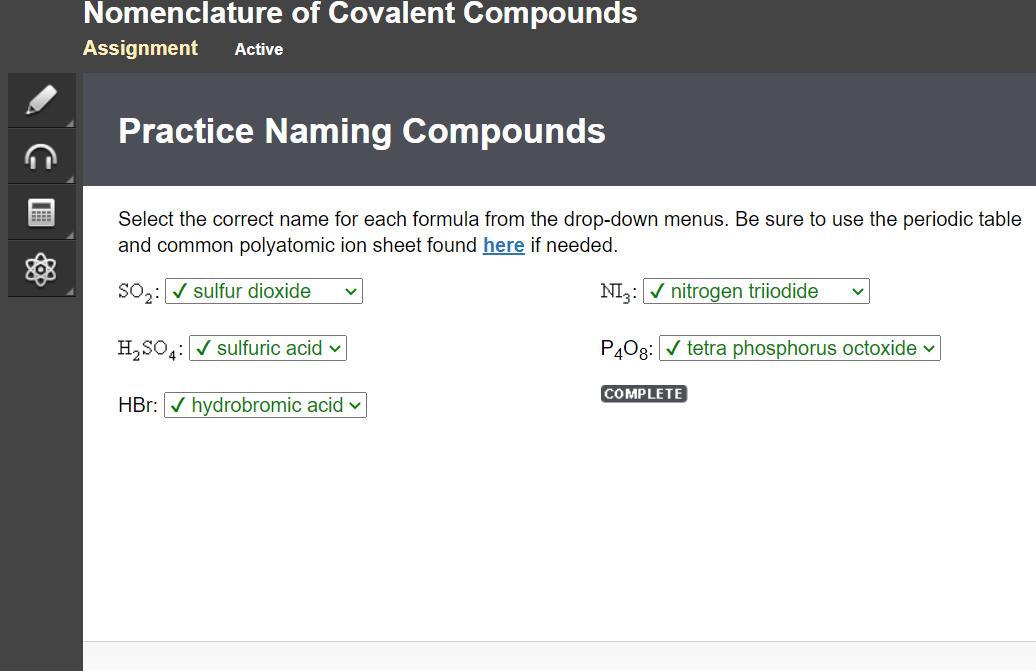
Select the correct name for each formula from the drop-down menus. Be sure to use the periodic table
and common polyatomic ion sheet found here if needed.
Answers
Answer:
here are the answers edge2020
Explanation:

Answer:
Here is 2/5 and the next slide answers too!
Explanation:
Hope it helps!!

what appearance of an acidic analyte solution containing phenolphthalein indicates the endpoint of titration with a basic solution? select one: a burst of pink color that disappears upon stirring. a persistent dark pink color throughout the solution. a persistent pale pink color throughout the solution. a burst of pink color that darkens upon stirring.
Answers
Phenolphtalein is chosen because it changes color in a pH range between 8.3 - 10. It will appear pink in basic solutions and clear in acidic solutions.
What is Titration ?A titration is a method where the concentration of an unknown solution is ascertained by comparing it to a solution of known concentration. The analyte (the unknown solution) is typically added in a known amount to the titrant (the known solution) from a buret until the reaction is finished.
When dissolved in acidic or basic liquids, phenolphthalein is colourless. As a result, at the equivalence point of this titration, it ought to turn from colourless to purple. The endpoint denotes our best guess as to the location of the equivalence point.Titration is a typical analytical chemistry method for figuring out a solution's concentration by progressively adding a solution with a known concentration.Learn more about Titration here:
https://brainly.com/question/186765
#SPJ4
Which of these is a compound? оо CO2 o 02 O CO
Answers
The answer is CO2 because 02 is a oxygen molecule and CO is a carbon monoxide!
The half-life of carbon-14 is 5,730 years. Dating organic material by looking for C-14 can’t be accurately done after 50,000 years. Suppose a fossilized tree branch originally contained 4. 30 grams of C-14. How much C-14 would be left after 50,000 years? Use the formula N = N0. A tree branch that originally had 4. 3 grams of carbon-14 will have grams after 50,000 years.
Answers
The formula for calculating the amount of carbon-14 left after a given number of years is N = N0 * (0.5)^(t/t_1/2).
Carbon-14 dating, also called radiocarbon dating, method of age determination that depends upon the decay to nitrogen of radiocarbon (carbon-14). Carbon-14 is continually formed in nature by the interaction of neutrons with nitrogen-14 in the Earth’s atmosphere; the neutrons required for this reaction are produced by cosmic rays interacting with the atmosphere.
where:
N is the amount of carbon-14 left after t years
N0 is the original amount of carbon-14
t_1/2 is the half-life of carbon-14 (5,730 years)
t is the number of years
In this case, we have:
N = 4.30 * (0.5)^(50,000/5,730) = 0.016796875
Therefore, after 50,000 years, there would be 0.016796875 grams of carbon-14 left in the fossilized tree branch. This is less than 0.1% of the original amount, so carbon-14 dating cannot be used to accurately determine the age of the tree branch.
To know more about half-life, click here:-
https://brainly.com/question/31666695
#SPJ11
How many moles are in 3.45g of KCI ?
Answers
0.0463 mol KCl
General Formulas and Concepts:Math
Pre-Algebra
Order of Operations: BPEMDAS
Brackets Parenthesis Exponents Multiplication Division Addition Subtraction Left to RightChemistry
Atomic Structure
Reading a Periodic TableUsing Dimensional AnalysisExplanation:Step 1: Define
3.45 g KCl
Step 2: Identify Conversions
Molar Mass of K - 39.10 g/mol
Molar Mass of Cl - 35.45 g/mol
Molar Mass of KCl - 39.10 + 35.45 = 74.55 g/mol
Step 3: Convert
Set up: \(\displaystyle 3.45 \ g \ KCl(\frac{1 \ mol \ KCl}{74.55 \ g \ KCl})\)Multiply/Divide: \(\displaystyle 0.046278 \ mol \ KCl\)Step 4: Check
Follow sig fig rules and round. We are given 3 sig figs.
0.046278 mol KCl ≈ 0.0463 mol KCl
Which of the following reactions in glycolysis is an aldose to ketose isomerization?
A) Enolase
B) Phosphoglycerate mutase
C) Phosphohexose isomerase
D) Aldolase
E) Glyceraldehyde-3-phosphate dehydrogenase
Answers
C) The reaction in glycolysis that involves an aldose to ketose isomerization is option Phosphohexose isomerase. Phosphohexose isomerase catalyzes the conversion of glucose-6-phosphate (an aldose) to fructose-6-phosphate (a ketose).
The reaction in glycolysis that involves an aldose to ketose isomerization is catalyzed by phosphohexose isomerase. This enzyme converts glucose-6-phosphate, which is an aldose sugar, into fructose-6-phosphate, which is a ketose sugar.
This is a crucial step in glycolysis as it allows for the rearrangement of the sugar molecule, facilitating further downstream metabolic processes.
The isomerization reaction occurs through the shifting of functional groups within the sugar molecule, resulting in the conversion from an aldose to a ketose.
This isomerization step ensures the proper progression of glycolysis and the generation of energy in the form of ATP.
To learn more about glycolysis here
https://brainly.com/question/30461189
#SPJ4
the action force always acts in the ____ direction as the reaction force
Answers
Answer:
opposite
Explanation:
Newtons law explains that for every action force there is an equal and opposite reaction.
A sample of Zn(s) is reacted with HCl(aq) to form hydrogen gas. The H2 gas bubbles out of aqueous solution and is collected in a 660 mL container at 411 Torr and 25. 0 C. How many grams of zinc reacted?
Answers
The mass of zinc that reacted is 0.480 g.
To determine the mass of zinc that reacted, we need to use the ideal gas law to calculate the number of moles of hydrogen gas produced in the reaction. We can then use the stoichiometry of the balanced chemical equation to find the number of moles of zinc that reacted, and convert that to grams using the molar mass of zinc.
The balanced chemical equation for the reaction of zinc with hydrochloric acid is:
Zn(s) + 2HCl(aq) → \(ZnCl_2(aq) + H_2(g)\)
From the equation, we can see that one mole of zinc reacts with two moles of hydrochloric acid to produce one mole of hydrogen gas.
First, we need to calculate the number of moles of hydrogen gas that was produced. We can use the ideal gas law to do this:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
We need to convert the pressure and volume to units that are consistent with the gas constant R, which has units of L·atm/mol·K. The given pressure of 411 Torr is equivalent to 0.541 atm, and the volume of the container is 660 mL, or 0.66 L. The temperature is given as 25.0°C, or 298.2 K.
Plugging in the values, we get:
(0.541 atm) (0.66 L) = n (0.0821 L·atm/mol·K) (298.2 K)
Solving for n, we get:
n = 0.0147 mol \(H_2\)
Since the stoichiometry of the balanced chemical equation tells us that one mole of zinc reacts with one-half mole of hydrogen gas, the number of moles of zinc that reacted is half of the number of moles of hydrogen gas:
n(Zn) = 0.5 × n(\(H_2\)) = 0.00735 mol Zn
Finally, we can convert the moles of zinc to grams using the molar mass of zinc, which is 65.38 g/mol:
m(Zn) = n(Zn) × M(Zn) = 0.00735 mol × 65.38 g/mol = 0.480 g Zn
Therefore, the mass of zinc that reacted is 0.480 g.
Know more about zinc here:
https://brainly.com/question/24527005
#SPJ11
How is water different from most substances during phase changes?
Answers
Water different from most substances during phase changes because water changes into gaseous water or steam
Those portion of a system that are physically distinct and mechanically separable from other portion of the system are called phases and a phase change is occurring the liquid water is changing to gaseous water or steam and on molecular level the intermolecular forces between the water molecule are decreasing the heat is providing enough energy for the water molecule to overcome these attractive forces
Know more about water
https://brainly.com/question/24131074
#SPJ1
Describe how the proton, electron and neutron were experimentally discovered.
Answers
Define first ionisation energy.
Answers
Answer:
the energy needed to remove the outermost, or highest energy, electron from a neutral atom in the gas phase.
Explanation:
What is the number of protons plus the number of neutrons equal to?
A. atomic number
b. atomic charge
c. atomic isotope number
d.mass number
Answers
Answer:
atomic number
Explanation:
The mass number of the atom (M) is equal to the sum of the number of protons and neutrons
What does the illustration indicate?

Answers
Answer: A
Explanation:
Which one is not a long-term environmental change?
Pollution
Swimming
Deforestation
Climate Change
Answers
Answer:
Swimming
Explanation:
Pollution is almost irreversible and takes forever to fix. deforestation leads to bad air quality and the erosion of the ground. climate change can melt polar caps or for instance freeze texas.
the diameter of a sodium atom, na, is larger than the diameter of a sodium ion, na , because the sodium ion has
Answers
Na+ has one fewer electron than Na, making it smaller and possessing a higher effective nuclear charge.
The fact that sodium ions have just two electron shells makes them smaller than sodium atoms for the main reason (the atom has three). Because they both have the same number of nucleons, the Na+ ion and Na atom have the same nuclear charge. Na+, on the other hand, has a smaller nuclear charge than Na because it has one fewer electron. Na+ is therefore smaller than Na because there is more attraction on the electrons that are already there. A sodium ion's size is smaller than that of a sodium atom.
Learn more about Nuclear Charge
brainly.com/question/17102072
#SPJ4
c) You pour a purple potassium permanganate solution (KMnO4) with a concentration of
2 mol/L in different volumetric flasks-100 mL/ 250mL/ 500ml - please calculate the
moles involved of each flask.
Answers
Answer:
Explanation:.
Which of the following best describes the function of the nucleus?
The nucleus controls the cell and contains DNA.
The nucleus transports materials inside the cell.
The nucleus holds the parts of the cell together.
The nucleus stores materials like food and waste.
Answers
Answer:
A
Explanation:
the nucleus has all the information about the cell and has the DNA
The function of the nucleus is that the nucleus controls the cell and contains DNA. Hence option A is correct.
What is DNA?DNA stands for deoxyribonucleic acid. DNA is defined as the molecule that contains the genetic material necessary for a creature to develop and function. Adenine (A), cytosine (C), guanine (G), and thymine (T) are the four different types of smaller chemical molecules together known as nucleotide bases that make up the linear molecule DNA .
Nucleus are defined as an organelle with two membranes that houses the genetic material and other instructions needed for cellular functions. A positively charged nucleus and a cloud of negatively charged atoms make form an atom.
Thus, the function of the nucleus is that the nucleus controls the cell and contains DNA. Hence option A is correct.
To learn more about DNA, refer to the link below:
https://brainly.com/question/264225
#SPJ6
please help me, thank you

Answers
Answer:
it's C
Explanation:
Yes
Answer:
It's neither 3 or 4 but I'm a little leaning to 4 as a right answer
what are the disruption of carbon cycle
Answers
Answer:
Human activities have a tremendous impact on the carbon cycle. Burning fossil fuels, changing land use, and using limestone to make concrete all transfer significant quantities of carbon into the atmosphere.
how does stem apply to science?
(i swear if yall just answer for points ima lose it)
Answers
STEM is an acronym meaning
Science
Technology
Engineering
Mathematics
One of the main focuses of STEM courses is on science, so you will be learning about various types of science
This is an approach at learning to have students learn all four types of subjects in one class, based on real world applications.
which solution has no effect on litmus?
1) acidic
2) basic
3) alkaline
4) neutral
Answers
Hope this help
A sanitation engineer in Portland, Maine sends a water sample to your lab, and wants to know if there are lactose-fermenting microbes in the sample (their presence would indicate contamination with human waste). How might you determine if these microbes are present or not from this mixed-microbe specimen?
Answers
Answer:
See the answer below
Explanation:
The first thing would be to isolate the bacteria in the water sample using appropriate isolation methods. After obtaining a pure culture of each isolate, a lactose broth fermentation test is then used to check for the isolates that are able to ferment lactose.
A sterile lactose broth containing an indicator (preferably, phenol red) is placed in different sterile test tubes and the pure culture of each isolate is aseptically introduced into each test tube. The test tubes are then incubated for 18 to 24 hours at 35 to 37 \(^oC\).
A change in the color of the indicator from red to yellow will give an indication of lactose fermentation by the organisms.
