Why is this considered a theory instead of a hypothesis or a law?
(When a dangerous microscopic organism is transferred from one person to many people,
Answers
Answer:
This is considered a hypothesis because it hasn't been confirmed. It is just a guess.
Explanation:
A hypothesis is a limited explanation of a phenomenon; a scientific theory is an in-depth explanation of the observed phenomenon. Law is a statement about an observed phenomenon or a unifying concept, according to Kennesaw State University. However, Newton's law doesn't explain what gravity is, or how it works.
Answer:
c
Explanation:
A. It is a statement of fact about how nature works instead of an explanation.
B. It provides an explanation for many observations and accepted hypotheses.
C. It describes how to test a possible explanation of one specific relationship.
D. It has not changed over time even though new technology has been developed.
Related Questions
briefly cite the differences between the recovery and recrystallization processes
Answers
1. Definition: Recovery is a process that occurs at relatively low temperatures and involves the removal of some of the effects of deformation, such as dislocations, without a significant change in the microstructure. Recrystallization, on the other hand, is a higher-temperature process that leads to the formation of new strain-free grains within the material.
2. Temperature: Recovery typically occurs at temperatures below the recrystallization temperature, whereas recrystallization occurs above this temperature. The specific temperature range for both processes depends on the material and its composition.
3. Microstructural Changes: In the recovery process, dislocations within the deformed material rearrange and reduce their density, leading to a decrease in stored energy and an increase in ductility. Recrystallization, however, involves the nucleation and growth of new grains, replacing the deformed structure with a more equiaxed and strain-free microstructure.
4. Time Scale: Recovery is a relatively fast process compared to recrystallization. It occurs within shorter time frames and can be completed within minutes or hours. Recrystallization, on the other hand, is a slower process that may require several hours or days to complete.
5. Mechanical Properties: Recovery primarily affects the mechanical properties of the material by reducing its stored energy and increasing its ductility. Recrystallization, in addition to improving ductility, also leads to a reduction in strength and hardness due to the formation of new, strain-free grains.
It's important to note that recovery and recrystallization are interrelated processes that can occur simultaneously or sequentially, depending on the material and deformation conditions.
Think of a time you might have used the scientific method without even realizing it.
Answers
Answer:
when i exist
Explanation:
The electron configuration of nitrogen (N) is
1s²2s²2p³
1s 2s 2p
1s²2s²2p5
1s²2s²2p
Answers
Answer:
The electron configuration of nitrogen (N) is 1s²2s²2p³ . The electron configuration of an atom describes the distribution of electrons among different energy levels and orbitals. The first number in the electron configuration represents the principal energy level, while the letter represents the sublevel (s, p, d, or f). The superscript number represents the number of electrons in that sublevel .
The electron configuration of nitrogen (N) is 1s²2s²2p³. The correct option is 1.
An atom's electron configuration specifies how its electrons are dispersed throughout the various energy levels and orbitals.
The electron configuration of nitrogen (N), which has an atomic number of 7, is 1s²2s²2p³.
Nitrogen has a total of 7 electrons in this arrangement. The first energy level (1s) is completely occupied by two electrons, and the second energy level (2s) is also completely occupied by two electrons.
The remaining three electrons are in the 2p orbital. The 2p orbital is broken down into three suborbitals: 2px, 2py, and 2pz. Each suborbital may carry a maximum of two electrons, accounting for the three electrons in nitrogen's 2p³ structure.
Thus, the correct option is 1.
For more details regarding electronic configuration, visit:
https://brainly.com/question/29184975
#SPJ5
Your question seems incomplete, the probable complete question is:
The electron configuration of nitrogen (N) is
1s²2s²2p³1s 2s 2p1s²2s²2p51s²2s²2parrange the following elements in order of increasing atomic size. k, na, mg, cs, cl
Answers
The order of increasing atomic size for the given elements is: Cl < Na < Mg < K < Cs
The atomic size of an element is determined by the distance between the nucleus and the outermost electron shell. As we move down a group in the periodic table, the atomic size increases due to the addition of new energy levels. As we move across a period, the atomic size decreases due to the increasing nuclear charge that attracts the electrons closer to the nucleus.
Therefore, to arrange the given elements in order of increasing atomic size, we need to consider their position in the periodic table.
The elements given are: K (potassium), Na (sodium), Mg (magnesium), Cs (cesium), and Cl (chlorine).
Starting from the smallest atomic size and moving towards the largest, we get:
1. Cl (chlorine) - Chlorine belongs to group 17 or the halogen group. As we move across a period from left to right, the atomic size decreases due to the increasing nuclear charge that attracts the electrons closer to the nucleus. Therefore, chlorine has the smallest atomic size among the given elements.
2. Na (sodium) - Sodium belongs to group 1 or the alkali metal group. As we move down a group from top to bottom, the atomic size increases due to the addition of new energy levels. Therefore, sodium has a larger atomic size than chlorine.
3. Mg (magnesium) - Magnesium belongs to group 2 or the alkaline earth metal group. Like sodium, it also has a larger atomic size than chlorine due to the addition of a new energy level.
4. K (potassium) - Potassium also belongs to group 1 or the alkali metal group, like sodium. However, it has a larger atomic size than sodium because it is located lower in the same group.
5. Cs (cesium) - Cesium belongs to the same group as potassium, but it has the largest atomic size among the given elements. This is because it is located at the bottom of the group and has the maximum number of energy levels.
Therefore, the order of increasing atomic size for the given elements is:
Cl < Na < Mg < K < Cs
To know more about atomic size, refer
https://brainly.com/question/30395515
#SPJ11
how is a limiting reactant problem different from other stoichiometry problems? (what is your clue that it is a limiting reactant problem?)
Answers
A limiting reactant problem is a type of stoichiometry problem that involves determining which reactant in a chemical reaction will be completely consumed, and therefore limit the amount of product that can be formed.
The key clue that a problem is a limiting reactant problem is the presence of information about the amounts or masses of two or more reactants that are involved in a chemical reaction. In a limiting reactant problem, you are typically given the amounts of two or more reactants, and asked to determine the amount of product that can be formed.
To solve a limiting reactant problem, you must first determine the balanced chemical equation for the reaction, and then use stoichiometry to calculate the theoretical amount of product that can be formed from each reactant. The reactant that produces the smallest amount of product is the limiting reactant, because it will be completely consumed in the reaction, while the other reactant(s) will be left over.
The calculation of the limiting reactant and the amount of product produced from it is what sets a limiting reactant problem apart from other stoichiometry problems. In other types of stoichiometry problems, you may be given the amount of a single reactant or product, and asked to find the amount of another reactant or product using stoichiometry.
To learn more about Limiting reactant :
https://brainly.com/question/28938721
#SPJ4
What are the chemical names of these ionic compounds?
Select the correct answer from each drop-down menu.
Answers
mole to mole rations

Answers
Based on the given equation: N₂+ 3 H₂ → 2 NH₃, the mole-to-mole ratios are:
a. N₂/H₂ - 1 : 3
b. N₂/NH₃ - 1 : 2
c. H₂/NH₃ - 3 : 2
KNO3
Based on the given equation: 8 H₂ + S₈ → 8 H₂S,the mole-to-mole ratios are:
a. H₂/H₂S - 1 : 1
b. H₂/S₈ - 8 : 1
3. Based on the given equation: 2 H₂ + O₂ → 2 H₂O
a. The H₂/H₂O mole-to-mole ratio is 1 : 1
b. Suppose you had 20 moles of H₂ on hand and plenty of O₂, the number of moles of H₂O you could make is 20 moles of H₂O.
C. The O₂ / H₂O mole to mole ratio is 1 : 2
d. Suppose you had 20 moles of O₂ and enough H₂, the number of moles of H₂O you could make is 40 moles.
What is the mole ratio of a reaction?The ratio of the mole quantities of any two compounds present in a balanced chemical reaction is known as the mole ratio. A comparison of the ratios of the molecules required to accomplish the reaction is given by the balancing chemical equation.
Learn more about mole ratio at: https://brainly.com/question/26023
#SPJ1
The heat of vaporization for ethanol is 0.826 kJ/g . Calculate the heat energy in joules required to boil 31.95 g of ethanol.
Answers
The heat energy required to boil 31.95 g of ethanol is 26,403.7 Joules.
To calculate the heat energy required to boil a given mass of ethanol, we can use the heat of vaporization and the mass of the substance.
The heat of vaporization of ethanol = 0.826 kJ/g
Mass of ethanol = 31.95 g
To convert the heat of vaporization from kJ/g to J/g, we multiply it by 1000:
Heat of vaporization = 0.826 kJ/g × 1000 J/kJ = 826 J/g
Now we can calculate the heat energy required to boil 31.95 g of ethanol:
Heat energy = Heat of vaporization × Mass
Heat energy = 826 J/g × 31.95 g
Using a calculator, we find:
Heat energy = 26,403.7 J
Therefore, the heat energy required to boil 31.95 g of ethanol is 26,403.7 Joules.
learn more about heat energy here:
https://brainly.com/app/ask?q=define+heat+of+vaporization
#SPJ11
Balance the chemical equation : CH₄ + O₂ → CO₂ + H₂O
Answers
Answer:
balanced equation: CH₄ + 2O₂ → CO₂ + 2H₂O
Explanation:
Given sample: CH₄ + O₂ → CO₂ + H₂O
The carbon is same, so no need to change. Hydrogen 2 less on left side so putted "2" before H₂O = "2H₂O"so now, there is total 4 oxygen on left side to balance put 2 before right side oxygen like this "2O₂"changes applied: CH₄ + 2O₂ → CO₂ + 2H₂O
HELP PLS THANK YA!!!
Is Cu(OH)2 a covalent bond , an ionic bond or both?
Answers
The bond between the Cu²⁺ and OH⁻ ions is, well, ionic. However, the bond between the O and the H in the OH⁻ ion is covalent. So Cu(OH)₂ is, as a whole, an ionic compound, but it contains or comprises both ionic and covalent bonds.
why is using only clean glassware important?
Answers
Good laboratory technique demands clean glassware because the most carefully executed piece of work may give an erroneous result if dirty glassware is used. In all instances, glassware must be physically and chemically clean and in many cases, it must be bacteriologic-ally clean or sterile.
Calculate the equilibrium constant of the reaction below if the pressures are 1.0atm, 2.0 atm, and 1.0 atm respectively. PCl3 + Cl2 <--> PCl5
Answers
Answer:
K = 0.5
Explanation:
Based on the reaction:
PCl₃ + Cl₂ ⇄ PCl₅
The equilibrium constant, K, is defined as:
K = P PCl₅ / P PCl₃ * P Cl₂
Where P represent the pressure at the equilibrium for each one of the gases involved in the equilibrium.
As:
P PCl₅ = 1.0atm
P PCl₃ = 1.0atm
P Cl₂ = 2.0atm
K = 1.0atm / 1.0atm * 2.0atm
K = 0.5If 359 mL of a gas were contracted to only 269 mL, what would be the initial
temperature of it if it measured 422 K after the change?
Answers
A gas that initially had a temperature of 422 K and was reduced to only 269 mL would now have an initial temperature of 563.19 K.
Volume at start: 359 mL
Volume completed: 269 mL
422 K is the final temperature.
Temperature at the start =?
Charle's Law can be used to resolve the issue. Charles Law states that with constant pressure and molecular number, the volume of a given amount of a gas is directly proportional to its temperature.
V₁/T₁ = V₂/T₂
Initial volume: V1
Initial temperature is T1.
Final volume is V2.
T2 = Actual temperature.
We will now enter the values into the formula.
V₁/T₁ = V₂/T₂
T₁ = V₁T₂ / V₂
T1 = 359 ml 269 ml / 422 K
T1=151498 ml.K/ 269 ml
T₁ = 563.19 K
To know more about Charle's law visit
https://brainly.com/question/16927784
#SPJ4
The weight of 100g H2 and 100 g He is same yet the number of elements is not same.
Answers
Answer:
The mole and atonmicity of both the gases are different, the number of atoms is not same.
Explanation:
The number of atoms in a molecule (compound) depends on mole number and atomicity.
↬ Mole of 100 g H₂ = 100g ÷ 2u = 50 mole
∴Number of atoms in 100 g H₂
= 2 x 50 x 6.022 × 10²³
= 6.022 x 10²⁴ atoms
↬ Mole of 100 g He = 100g ÷ 4u = 25 mole
∴ Number of atoms in 100 g He
= 1 × 25 × 6.022 × 10²³
= 150.55 × 10²³
= 1.5055 x 10²⁵ atoms
Thus, The mole and atonmicity of both the gases are different, the number of atoms is not same.
-TheUnknownScientist 72
What is the mass of 0.45 mol of ammonium sulfate, (NH4)2SO4?
Answers
Answer:
59.46 g
Explanation:
To answer this question, the molecular weight of ammonium sulfate must be computed. To accomplish this, the weights of the individual elements must be noted.
N=14.01\(\frac{g}{mol}\)
H=1.01\(\frac{g}{mol}\)
S=32.07\(\frac{g}{mol}\)
O=16.00\(\frac{g}{mol}\)
To compute the molecular weight:
\(2[14.01\frac{g}{mol}+4(1.01\frac{g}{mol})]+32.07\frac{g}{mol}+4(16.00\frac{g}{mol})=132.14\frac{g}{mol}\)
To calculate the mass:
\(0.45 mol(\frac{132.14g}{1mol})=59.463g\)
What is the minimum number of grams of CO require
produce 88 grams of CO2?
A) 28 g
B) 88 g
C) 64 g
D) 56 g
Answers
Answer:
The minimum number of grams of CO require
produce 88 grams of CO2 is 64 g.
73. Arrange the following aqueous solutions in order of increasing boiling points: 0. 300m C6H12O6, 0. 110m K2CO3, and 0. 050m Al(ClO4)3 A) C6H12O6 < K2CO3
Answers
The aqueous solutions can be arranged in increasing boiling point order as follows: 0.050m Al(ClO4)3 < 0.110m K2CO3 < 0.300m C6H12O6.
The boiling point of a solution is influenced by the concentration of solute particles in the solution. The greater the concentration of solute particles, the higher the boiling point. In this case, we are comparing the boiling points of three different aqueous solutions.
The solution with the lowest boiling point is 0.050m Al(ClO4)3. This is because Al(ClO4)3 is an ionic compound that dissociates into multiple ions in water, thereby increasing the number of solute particles. Higher concentration of solute particles raises the boiling point.
The solution with the next higher boiling point is 0.110m K2CO3. K2CO3 is also an ionic compound and dissociates into two ions in water. Although the concentration is higher compared to Al(ClO4)3, it is lower than that of C6H12O6.
The solution with the highest boiling point is 0.300m C6H12O6. C6H12O6, which is glucose, is a molecular compound and does not dissociate into ions in water. Therefore, it has the lowest concentration of solute particles among the given solutions, resulting in the lowest boiling point.
Hence, the correct order of increasing boiling points is 0.050m Al(ClO4)3 < 0.110m K2CO3 < 0.300m C6H12O6.
learn more about boiling point here
https://brainly.com/question/2153588
#SPJ11
1. If I have 3 identical books that all have a length of 25cm, a
width of 15cm, and are 11cm tall, what would be the volume of
all 3 books?
Answers
Answer:
12,375 cm³
Explanation:
v=l×w×h
Since there are 3 books the equation would then be
v=3(l×w×h)
v=3(25×15×11)
v=3(4,125)
v=12,375 cm³
PLEASE You need to make a 0.23 M solution of NaCl using a 250 mL volumetric flask. How many grams of NaCl do you need? Round to the hundredths place (0.01)
Answers
Answer:
Easy. You turn 250 mL into Liters which comes out to be 0.25. We now need moles so you multiply 0.25 L and 0.23 M of NaCl and you get 0.058 moles of NaCl. Now you need to turn moles into grams so 0.058 moles x 58.44 (NaCl molar mass) /1 mol and you get 3.39 grams of NaCl.
Explanation:
I might be wrong so good luck.
Heat energy is _________.
Answers
ANSWER:Atoms
Explanation:
when heat energy is the result of movement of tiny particles called atoms. molecules or ions in solid liquids and gases heat energy can be transferred from one object to another the transfer or flow due to the difference in temperature between the two objects is called heat
MARKING BRAINLIEST!
Many processes occur in the digestive system. Which process is best classified as a physical change?
A. digestive enzymes breaking down proteins into smaller fragments
B. teeth grinding an almond into smaller pieces in the mouth
C. bacteria converting lactose into simple sugars in the intestines
D. saliva converting the starch molecules in crackers into simpler sugars
Answers
Answer:
Teeth gridning
Explanation:
BC YOUR GRINDING IT
What element has 16 electrons, 16 protons, and 16 neutrons
Answers
Answer:
The element that has 16 electrons, 16 protons, and 16 neutrons is sulfur (S)
Explanation:
The atomic number tell you the number of protons.
The atomic configuration tell you the number of electrons.
The mass number minus the protons tell you the number of neutrons.
Calculate the density of pentane with a mass of 47 grams and a volume of 75 mL.
Answers
Answer:
The answer is
0.63 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
From the question
mass = 47 g
volume = 75 mL
The density is
\(density = \frac{47}{75} \\ = 0.626666666...\)
We have the final answer as
0.63 g/mLHope this helps you
2. Which of the following shows the correct, balanced equation for the reaction shown below?
CH4+0₂H₂O + CO₂
O CH, + O, → CHO
O CH4 +20₂ → 2 H₂O + CO₂ OCH, + O2 → H2O +CO,
OCH4 +0₂ → CH₂0
Answers
The balanced equation is \(\text{CH}_{4}+2\text{O}_{2} \longrightarrow \text{CO}_{2}+2\text{H}_{2}\text{O}\).
The correct balanced chemical equation is CH₄ + 2 O₂\(\rightarrow\) CO₂ + 2 H₂O.
What is chemical equation?Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
The first chemical equation was put forth by Jean Beguin in 1615.By making use of chemical equations the direction of reaction ,state of reactants and products can be stated. In the chemical equations even the temperature to be maintained and catalyst can be mentioned.
Learn more about chemical equation,here:
https://brainly.com/question/29130807
#SPJ5
i’m too dumb for school.
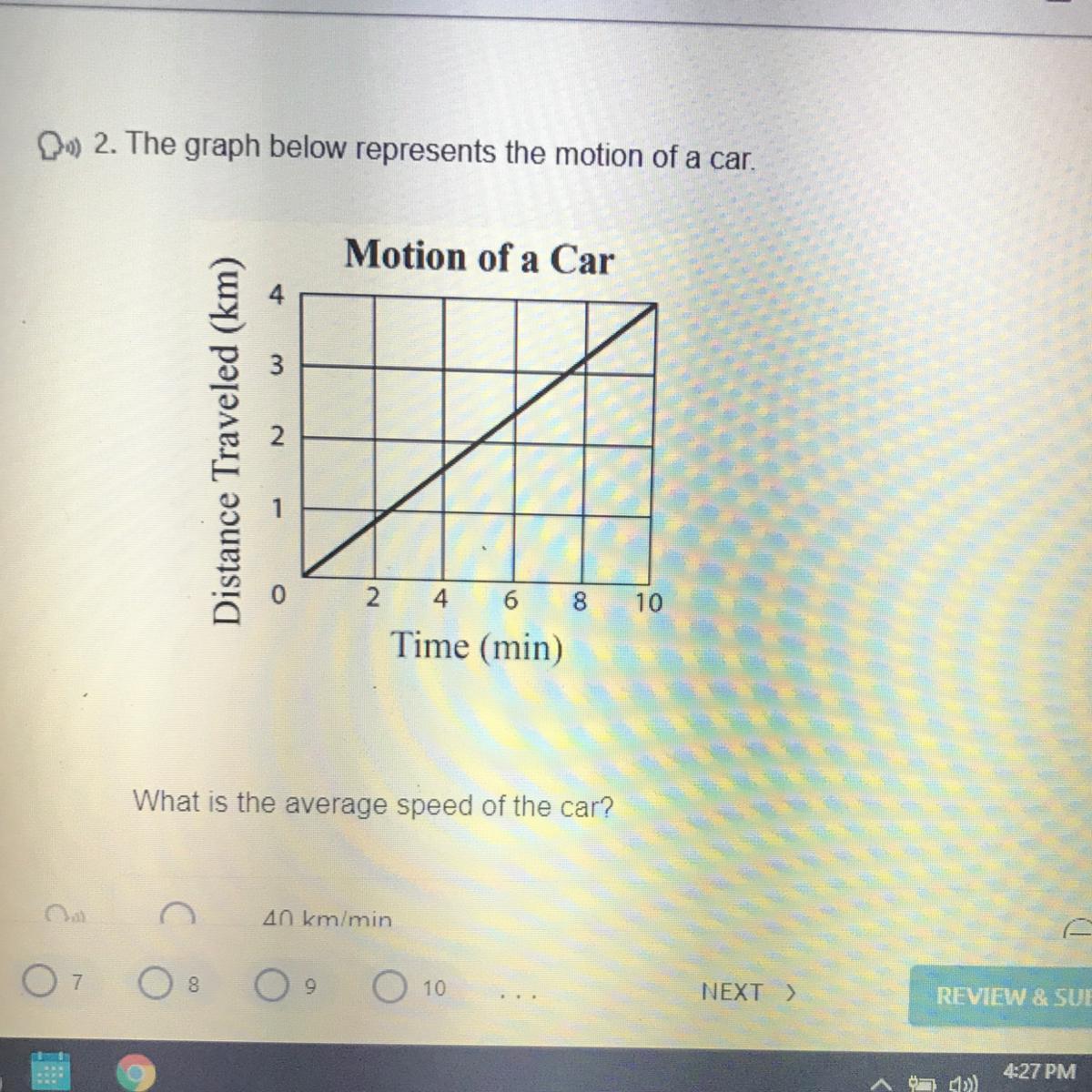
Answers
Answer:
every two minutes the car moves 1km
Explanation:
how do to draw resonance structures organic chemistry
Answers
Here's a step to draw resonance structures in organic chemistry:
There must be genuine Lewis structures in every resonance structure. (Remember that all the guidelines that apply to Lewis structures still hold true here.) The only difference in resonance structures' atom connection and electron configuration. (Electrons move; atoms NEVER do.)
The electron count and net charge are identical for all resonance configurations. (An atom's formal charge may differ, but the net charge, which is the total of all charges, must be the same.)
Only electrons and lone pair electrons (NEVER move bonds!) can be transferred by employing one of the following three transformations to move electrons from a region with a greater electron density to one with a lower electron density:
a π bond forms another π bond;a π bond forms the lone pair electrons; andlone pair electrons forms a π bond.To depict the motion of the electrons in the "original" resonance structure, use curved arrows. By following the arrows, the "new" resonance structure should be a "product" that is automatically created.
Determine the "new" structure's formal charge and note any non-zero formal charges.
example attached below
Only the electrons can move around in this example because there is only one link and no lone pairs. The low electron density region, or carbocation, is present next to the bond. As a result, it makes sense to transfer the electrons to the position next to the carbocation to create another bond, which results in the "new" structure. Here, the two resonance structures are identical.
Learn more about resonace at https://brainly.com/question/13564486
#SPJ4

consider a soluble salt in which the absolute value of the heat of hydration is less than the absolute value of the lattice enthalpy. what are the signs of standard gibbs energy, enthalpy and entropy of precipitation? select the words positive, zero, negative, or unknown in each of the boxes when adding a solid salt to water.
Answers
When a soluble salt is present in which the absolute value of the heat of hydration is less than that of the value of the lattice enthalpy, the signs of standard Gibbs energy, enthalpy, and entropy of precipitation would be positive, zero, and negative respectively.
Entropy is a thermodynamic property which is defined as the measure of the degree of randomness present in a system. It is represented by "S". Specifically, it describes the number of possible arrangements of a system that are consistent with its macroscopic state functions (e.g. pressure, temperature and volume). Greater the number of possible arrangements, the higher the entropy.
Changes in temperature, pressure, and the number and types of particles present in a system affects the entropy. By increasing the temperature or addition of particles to a system increases entropy, while on decreasing the temperature or decreasing the number of particles decreases entropy.
In a soluble salt, when absolute value of heat of hydration is less than absolute value of lattice enthalpy then the signs of standard gibbs energy, enthalpy and entropy of precipitation are positive, zero and negative respectively.
To know more about entropy here
https://brainly.com/question/31057236
#SPJ4
How many moles of a gas would occupy 22.4 Liters at 273 K and 1 atm?
Read bottom comment
Answers
Answer:
The following relationship makes this possible: 1 mole of any gas at standard temperature and pressure (273 K and 1 atm) occupies a volume of 22.4 L.
Explanation:
1 mole of any gas would occupy 22.4L of gas at standard temperature and pressure (STP) i.e at 273K and 1 atm.
The STP conditions refer to the standard temperature and pressure. Pressure values at 1 atmosphere and temperature at 0 ° C (or 273 K) are used and are reference values for gases. And in these conditions 1 mole of any gas occupies an approximate volume of 22.4 liters.
An ideal gas is characterized by three state variables: absolute pressure (P), volume (V), and absolute temperature (T). The relationship between them constitutes the ideal gas law, an equation that relates the three variables if the amount of substance, number of moles n, remains constant and where R is the molar constant of the gases:
P V = n R T
To know more about moles of gas here
https://brainly.com/question/32658876
#SPJ2
a brief description of atoms and how they relate to
molecules and compounds
Answers
Answer:
Explanation:
Atoms are the thing that make up molecules and compounds. Molecules contain two or more atoms and are held together by covalent bonds
Which of the following is not a benefit of using a scientific model?
А Models allow you to formulate hypotheses about processes.
B Models allow you to study processes that cannot be duplicated.
C Models are often simplified to help explain complex concepts.
D Models make it possible to duplicate the real thing.
Answers
Answer:
Benifits of scientific modeling
Explanation:
When students are engaged in scientific modeling, they are able to notice patterns and develop and revise representations that become useful models to predict and explain--making their own scientific knowledge stronger, helping them to think critically, and helping them know more about the nature of science.