why is it recommended to keep the reaction temperature low and the addition of nitric aci-dulfuric acid mixture out slowlt
Answers
It is recommended to keep the reaction temperature low and the addition of nitric acid sulfuric acid mixture out slow because the reaction between the two is exothermic, which means it produces a lot of heat. The high temperature produced can result in an explosion, which can be dangerous.
The exothermic nature of the reaction causes the formation of nitronium ions, which act as an electrophile to nitrate the organic substrate. If the temperature is too high, the nitronium ions can form too fast, causing the reaction to run out of control. Additionally, the addition of the nitric acid sulfuric acid mixture should be slow to avoid the formation of nitrogen dioxide gas.
Nitrogen dioxide is produced when the nitric acid reacts with atmospheric nitrogen oxide. This can lead to a brown or yellow coloration of the reaction mixture and, in high concentration, can be toxic. By adding the mixture slowly, the concentration of nitrogen dioxide is reduced, making the reaction safer.
In conclusion, it is crucial to keep the reaction temperature low and add the nitric acid sulfuric acid mixture slowly to prevent an explosion from the high temperature produced by the exothermic reaction. The slow addition of the mixture also reduces the concentration of nitrogen dioxide, making the reaction safer.
To know more about Exothermic refer here:
https://brainly.com/question/3971357#
#SPJ11
Related Questions
What structure actively moves water through a sponge?
Answers
Answer:
Answer is flagella
Explanation:
I hope it's helpful!

Precipitate-forming reactions would be most appropriate for identifying the presence of?
Answers
Precipitate-forming reactions would be most appropriate for identifying the presence of whether an element is present in the solution.
What is precipitate forming reaction?In a precipitation reaction, dissolved chemicals will get combined to form one or more solid products. These kinds of reactions, which are also known as double displacement, double replacement, or metathesis reactions which are frequently taken place through the aqueous solutions and involved in the exchange of ions between ionic compounds.
Through the precipitation reactions, the presence of element will be confirmed and analyzed. The presence of lead in water sources could be checked by adding the chemical and looking for the appearance of a precipitation whether it should be at instant , and the chemical combines with lead to generate a precipitate.
To learn more about precipitate forming reaction,
brainly.com/question/9052471
#SPJ4
35. a
When aqueous iron (III) chiondes added to aqueous potassium iodide a chemical con
ours and lodine is formed
Which statement is correct?
A todide sons are oxidised, they gain electrons in this reaction
lodide ions are oxidised, they lone electrons in this reaction
C trond) chionde is oxidised in this reaction
D Neither iodide ions nor iron (III) chlonde is ondised in this reachon

Answers
Scientific method quick check
Answers
Answer:
Quick you said:
-Purpose/Question
Ask a question.
-Research
Conduct background research. Write down your sources so you can cite your references. In the modern era, a lot of your research may be conducted online. Scroll to the bottom of articles to check the references. Even if you can't access the full text of a published article, you can usually view the abstract to see the summary of other experiments. Interview experts on a topic. The more you know about a subject, the easier it will be to conduct your investigation.
-Hypothesis
Propose a hypothesis. This is a sort of educated guess about what you expect. It is a statement used to predict the outcome of an experiment. Usually, a hypothesis is written in terms of cause and effect. Alternatively, it may describe the relationship between two phenomena. One type of hypothesis is the null hypothesis or the no-difference hypothesis. This is an easy type of hypothesis to test because it assumes changing a variable will have no effect on the outcome. In reality, you probably expect a change but rejecting a hypothesis may be more useful than accepting one.
-Experiment
Design and perform an experiment to test your hypothesis. An experiment has an independent and dependent variable. You change or control the independent variable and record the effect it has on the dependent variable. It's important to change only one variable for an experiment rather than try to combine the effects of variables in an experiment. For example, if you want to test the effects of light intensity and fertilizer concentration on the growth rate of a plant, you're really looking at two separate experiments.
-Data/Analysis
Record observations and analyze the meaning of the data. Often, you'll prepare a table or graph of the data. Don't throw out data points you think are bad or that don't support your predictions. Some of the most incredible discoveries in science were made because the data looked wrong! Once you have the data, you may need to perform a mathematical analysis to support or refute your hypothesis.
-Conclusion
Conclude whether to accept or reject your hypothesis. There is no right or wrong outcome to an experiment, so either result is fine. Accepting a hypothesis does not necessarily mean it's correct! Sometimes repeating an experiment may give a different result. In other cases, a hypothesis may predict an outcome, yet you might draw an incorrect conclusion. Communicate your results. The results may be compiled into a lab report or formally submitted as a paper. Whether you accept or reject the hypothesis, you likely learned something about the subject and may wish to revise the original hypothesis or form a new one for a future experiment.
The mass of 90 bean seeds is 15kg what is the mass of 1 bean seed
Answers
Answer:
1/6 kg
Explanation:
90 bean seeds is 15kg
1 bean seeds is 15/90 = 1/6 kg
Answer: 0.166 kg
90 bean seeds --> 15kg
1 bean seed --> 15/90 which is 0.166 kg
Brainliest pwease if it is correct!
ฅ ^ • ﻌ • ^ ฅ
dissolving a solute such as KOH in a solvent such as water results inA.) a decrease in the vapor pressure of the liquid B.) a decrease in the boiling point of the liquid C.) no change in the boiling point of the liquid D.) an increase in the melting point of the liquid
Answers
Dissolving a solute such as KOH in a solvent such as water results in- option A] a decrease in the vapor pressure of the liquid.
We can say that vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (here liquid) at a given temperature in a closed system. Since the equilibrium vapor pressure is more like an indication to a liquid's thermodynamic tendency to evaporate, as it relates to the balance of particles escaping from the liquid in equilibrium with those in a coexisting vapor phase. With this a substance with a high vapor pressure at normal temperatures is often referred to as commonly known as volatile. Therefore, we know that the pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the attractive interactions between liquid molecules become less significant in comparison to the entropy of those molecules in the gas phase, also increasing the vapor pressure along with it. Thus, we know that liquids with strong intermolecular interactions are likely to have smaller vapor pressures, and vice versa for weaker interactions.
To learn more about vapor pressure, click here:
brainly.com/question/11864750
#SPJ4
Estimate the boiling point of water in °C atop the Denali mountain (in Alaska). atmospheric pressure atop Denali is 657 torr;\DeltaΔH vap of water = 40.7 kJ/mol Enter to 2 decimal places.
Answers
The estimated boiling point of water atop Denali mountain is approximately -267.46°C.
First, we convert the given atmospheric pressure from torr to atm:
657 torr / 760 torr/atm ≈ 0.865 atm
Next, we use the Clausius-Clapeyron equation to relate the boiling point of a substance to its vapor pressure:
ln(P₂/P₁) = (ΔH_vap/R) * (1/T₁ - 1/T₂)
where P₁ and T₁ are the reference pressure and boiling point, P₂ is the reduced pressure atop Denali (0.865 atm), ΔH_vap is the enthalpy of vaporization of water (40.7 kJ/mol), R is the ideal gas constant (0.0821 L·atm/(mol·K)), and T₂ is the boiling point we want to determine.
We can assume the reference boiling point of water at normal atmospheric pressure (1 atm) to be 100°C or 373 K.
Solving the equation for T₂:
ln(0.865/1) = (40.7 kJ/mol / (0.0821 L·atm/(mol·K))) * (1/373 - 1/T₂)
0.865 = 4.95 * (1/373 - 1/T₂)
1/T₂ = (0.865 / (4.95 * 0.865)) + 1/373
1/T₂ ≈ 0.173 + 0.00268
1/T₂ ≈ 0.17568
T₂ ≈ 1/0.17568
T₂ ≈ 5.69 K
Converting back to Celsius:
T₂ ≈ 5.69 - 273.15 ≈ -267.46°C
Learn more about boiling point here:
https://brainly.com/question/29233996
#SPJ11
How many electrons in 23Na+?
How many neutrons in 23Na+?
Answers
Answer:
There are 12 neutrons and 11 electrons in 23Na+
Explanation:
An atomic number of 11 means this atom will have 11 protons. A mass number of 23 means 23 - 11 this atom will have 12 neutrons. Since this atom is neutral the positive protons must be equal to the negative electrons. This atom will have 11 electrons.
which statement is true concerning the Haber Process?
A.
It is a process for the synthesis of nitrogen gas.
B.
It is a process for the synthesis of ammonia.
c.
It is a process for the synthesis of methane.
D.
It is a process for the synthesis of carbon monoxide.
E.
It is a process for the synthesis of carbon dioxide.
Answers
Answer: the answer is B The Haber Process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the conditions used in the process. The process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia.
Explanation: Mark as brainliest if it helped!!
In the chemical reaction, calcium carbonate (CaCO3) was heated to form two new
substances: calcium oxide (Cao) and carbon dioxide (CO2).
CaCO3 + CaO + CO2
A student heated 24.8 g of CaCO3. After the reaction, they measured 13.9 g of Cao.
What was the mass of the CO2 gas that escaped during the reaction?
38.79
O 1.78 g
0 24.8 g
10.99
Answers
Answer: 10.99
Explanation: because you take the Cao 13.9 and take CO2 which is 10.99 and it makes 24.8 . Which is CaCO3.
Decomposition reactions are the breakdown of chemical species into simpler parts. Decomposition reactions typically require energy input.
The mass of the CO2 gas that escaped = 10.99 g.
What is a decomposition reaction ?Decomposition reactions are the breakdown of chemical species into simpler parts. Decomposition reactions typically require energy input.When one reactant breaks down into two or more products, this is referred to as a decomposition reaction. This is represented by the following general equation: AB A + B. The breakdown of hydrogen peroxide to water and oxygen is an example of a decomposition reaction, as is the breakdown of water to hydrogen and oxygen.When one reactant breaks down into two or more products, this is referred to as a decomposition reaction. It is denoted by the general equation: AB A + B. In this equation, AB represents the reactant that initiates the reaction, and A and B are the reaction products.Molecular mass of CaO = 56.07 g
CO\(_2\) = 44.01 g
the mass of the CO2 gas that escaped = 13.9 x 44.01/ 56.07
= 10.99 g.
To learn more about : Decomposition
Ref : https://brainly.com/question/14608831
#SPJ2
what do you mean by acceleration due to gravity of a body is 10 metre per second square

Answers
Answer:
If a body is accelerated by 10m/s^2, it means that the body's speed (or velocity to be precise) is increasing by 10m/s every second. That is, if the initial ...
Given the following atomic weights, calculate the molecular weight of water
H = 1.008 g/mol; O = 16.00 g/mol.

Answers
The molecular weight of water using the given atomic weights of H and O would be 18.02 g/mol.
Molecular weight calculationThe molecular weight of water can be calculated by adding the atomic weights of its constituent atoms. Water (H2O) consists of two hydrogen atoms (H) and one oxygen atom (O).
Molecular weight of water = (2 x atomic weight of hydrogen) + (1 x atomic weight of oxygen)
Given that the atomic weights of hydrogen and oxygen are 1.008 g/mol and 16.00 g/mol respectively:
Molecular weight of water = (2 x 1.008 g/mol) + (1 x 16.00 g/mol) Molecular weight of water = 18.02 g/molTherefore, the molecular weight of water is 18.02 g/mol.
More on molecular weights can be found here: https://brainly.com/question/15522377
#SPJ1
The ____________ controls the materials that move into and out of a cell.
cytoplasm
cell membrane
nucleus
Answers
Answer:
membrane
Explanation:
its semi permiable meaning it only lets certain things go in and out
Answer:
The cell membrane controls the materials that move into and out of a cell.
Explanation:
The cell membrane is selectively permeable to ions and organic molecules and controls the movement of substances in and out of cells.
I hope this helps!
An atom consits of electrons, protons
and
neutrons.where are these sub-atomic particles
located?
Answers
Answer:
electron: orbiting around the nucleus
proton: inside the nucleus
neutron: inside the nucleus
Answer:
the electrons orbit around the nucleus.
the nucleus is where the neutrons and protons are located
hope this helps

HELPPPP!!!! SCIENCE!!!!
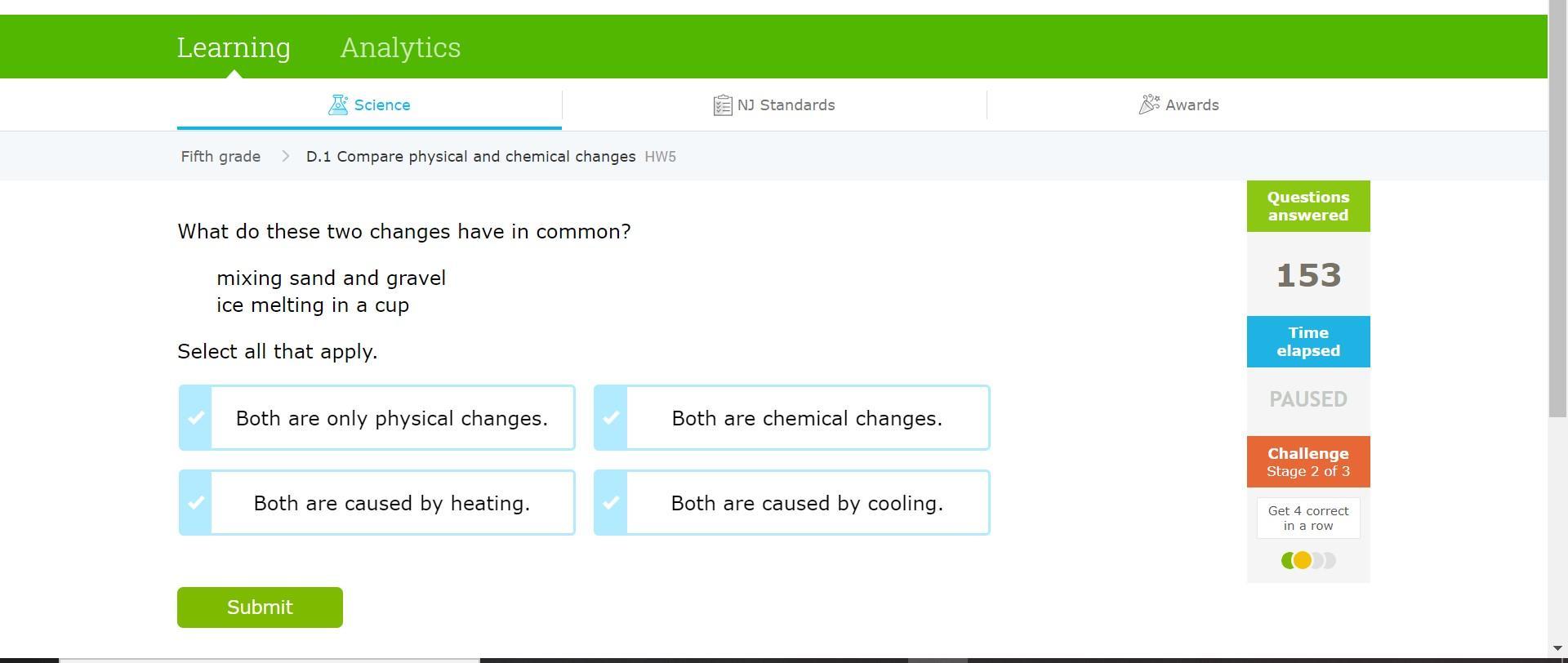
Answers
Answer:
Both are only physical changes
n experimenter found that 45.0g of a radioactive material goes through three half-lives. Calculate how many grams of the original material does she have left
Answers
An experimenter found that 45.0 g of a radioactive material goes through three half-lives. The question is to calculate how many grams of the original material does she have left.
Half-life: The half-life is the time it takes for half of the atoms in a sample of radioactive material to decay. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay. Therefore, the formula for calculating the half-life of a radioactive element is given by: mass of the original sample = remaining mass × 2^(number of half-lives)
Let's calculate the remaining mass of the sample. Remaining mass = Original mass / 2^(number of half-lives) Given, Original mass = 45 g and number of half-lives = 3
Therefore, Remaining mass = 45 / 2^(3)= 45/8= 5.625 g (approx)Hence, the experimenter will have 5.625 g of the original material left.
To know more about radioactive material refer here:
https://brainly.com/question/3542572#
#SPJ11
The rate of phosphorus pentachloride decomposition is measured at a PCI5 pressure of 0.015 atm and then again at a PCl5 pressure of 0.30 atm. The temperature is identical in both measurements. Which rate is likely to be faster?
Answers
The main answer to your question is that the rate of phosphorus pentachloride decomposition is likely to be faster at a PCl5 pressure of 0.30 atm.
This is because an increase in pressure typically leads to an increase in the number of collisions between molecules, which in turn increases the likelihood of successful collisions that result in reaction.
The rate of a chemical reaction is influenced by a number of factors, including temperature, concentration of reactants, and pressure. In this case, the temperature is held constant, so we can assume that it is not a contributing factor to the difference in rates.
Pressure, on the other hand, affects the behavior of gas molecules. At a higher pressure, there are more gas molecules in a given volume, which increases the frequency of collisions between molecules. This increase in collision frequency leads to a higher likelihood of successful collisions that result in reaction, which in turn increases the rate of the reaction. Therefore, the rate of phosphorus pentachloride decomposition is likely to be faster at a PCl5 pressure of 0.30 atm compared to a pressure of 0.015 atm.
For more information on phosphorus pentachloride visit:
https://brainly.com/question/29141612
#SPJ11
ipt Hydrogen and oxygen react to produce water. Which of the following is the correctly balanced chemical equation
for this reaction?
A. H + O2 + H2O
B. 2H2 + O2 + 2H2O
O c. H, + O2 + H 02
OD. 2H, + 202 + 4H, O
Answers
Answer:
H + O2 + H2O
Explanation:
what is the mass of 0.257 mol of calcium nitrate?
Answers
Answer: 42.14 g
Explanation: calcium nitrate - \(Ca(NO_{3} )_{2}\)
, the molar mass of calcium nitrate = 164 g
formula used = \(given mass/molar mass\)= no of molesmass required =\(0.257*164\)
=\(42.14\) g
Do you think Hugh would have been able to perform this experiment in his laboratory at home 20 years ago? Explain your answer.
Answers
Answer:scientists finished deciding the human genome in 2001. This research helped genetics analyze and islote sections of DNA for investigation. Without this information and the technology invented during the Human Genome Project, Hugh would not have been able to extract portions of his daughters DNA. Also, he wouldn’t have been able to see the genes that did not function properly in his daughters DNA.
Explanation: this is a right answer for PLATO.
What is the mass of 0.2 mole of sodium.
Answers
We know that 1 mole of sodium contains Avogadro number of atoms. - But we have to find the number of atoms in 0.2 mole of sodium. - Therefore 0.2 moles of sodium (Na) contains 12.046×1023atoms in it.
Particles with different charges:
a
are drawn toward each other
b
move closer together
c
attract each other
d
all of the above
Answers
Answer:
Your answer is D. All of the above
Explanation:
They are all the same answers just worded differently.
How many moles of Ca(OH)2 are needed to
neutralize three moles of HCI?
Answers
1.5 mole of Ca(OH)\(_2\) are needed to neutralize 2 moles of HCI. The mole idea is a useful way to indicate how much of a substance there is.
The mole idea is a useful way to indicate how much of a substance there is. Any measurement can be divided into two components: the magnitude in numbers and the units in which the magnitude is expressed. For instance, the magnitude is "2" and the unit is "kilogramme" when a ball's mass is determined to be 2 kilogrammes.
Ca(OH)\(_2\) + 2HCl → CaCl\(_2\) + 2H\(_2\)O
1 mole of Ca(OH)\(_2\) are needed to neutralize 2 moles of HCI.
so, 1.5 mole of Ca(OH)\(_2\) are needed to neutralize 2 moles of HCI.
To know more about mole, here:
https://brainly.com/question/31597231
#SPJ1
Which of the following describes a subalpine ecosystem?
It is found just below the tree line (lower elevations).
It has strong winds and cold weather.
It is found far above the tree line (higher elevations).
It is called the land of “extremes”.
Answers
Answer:
it is called land of (extreme)
Explanation:
please rank these 1 to most effective and 3 to least, it's for my final exam.

Answers
The rank based on the effect of the given factors on the calcification rate is as follows;
Add Ca²⁺Lower CO₂Add CO₃²⁻What is calcification rate?Calcification rate is the rate at which organisms such as corals, mollusks, and algae secrete calcium carbonate to form their hard skeletons or shells.
The calcification rate is influenced by various environmental factors such as temperature, pH, and the availability of calcium and carbonate ions.
Considering the effect of the given factors on the calcification rate;
Adding Ca²⁺ will be the most effective as it will increase the calcium carbonate saturation state, which can lead to increased calcification rates.Lowering CO₂ levels will be moderately effective, as it will also increase the saturation state, but to a lesser extent compared to adding Ca²⁺.Adding CO₃²⁻ will be the least effective, as it may not increase the saturation state enough to significantly improve calcification rates.Learn more about calcification at: https://brainly.com/question/31947524
#SPJ1
When you walk barefoot across a hot sidewalk, you feel the heat on your feet. Explain what’s happening with the molecules in both the sidewalk and your feet , and which direction thermal energy is moving
Answers
When you walk over a sidewalk with barefoot, your feet make contact with the surface, which conducts heat to your feet.Convection drives the majority of heat energy in the atmosphere.
Walking barefoot on warm sand causes what kind of heat transfer?Conduction transfers heat from heated sand to a bottoms of your feet.Heat is transferred by conduction when two items come into contact.Heat energy passes from the warmer to the colder of two materials when their temperatures differ.
What kind of heat transmission involves direct contact?conduction Three methods exist for transferring heat: conduction, convection, and radiation.Energy is transferred directly from one atom to another through conduction.
To know more about thermal energy visit:
https://brainly.com/question/11278589
#SPJ4
A chunk of dry ice, solid co2, disappears after sitting at room temperature for a while. There is no puddle of liquid. What happened?.
Answers
After a while at room temperature, a piece of dry ice, or solid CO2, vanishes without leaving a puddle of liquid behind.
What is solid CO2?Dry ice, which is a solid form of carbon dioxide, is available. When used for short-term refrigeration, CO2 is usually used because it frequently sublimates from the solid form to the gas state due to the fact that it does not have a liquid state at normal atmospheric pressure. Carbon dioxide that is solid is known as "dry ice." Due to its ice-like appearance and the fact that it truly sublimes rather than melting, dry ice appears to be made of ice. Because the solid does not first become a liquid when heated, it is known as dry ice.
What is solid CO2 used for?Dry ice, also known as solid carbon dioxide, is very cold (109 °F / 78 °C), and it is frequently used to cool vaccines, chill and freeze food, preserve blood and tissue samples, heat-treat metals, and even produce special effects like fog for events or stage plays. Because solid CO2 does not melt into a liquid when heated but instead transforms instantly into gas, it is referred to as dry ice. Sublimation is the name of this procedure.
To know more about Solid CO2 visit:
https://brainly.com/question/2726615
#SPJ4
In reaction A, each sodium atom gives one electron to a chlorine atom. In reaction B, an isotope of oxygen decays to form an isotope of nitrogen. Which statement best describes the energy that is released per gram by these reactions?
Reaction A releases more energy than reaction B releases.
Reaction B releases more energy than reaction A releases.
Reaction A and reaction B release equal amounts of energy.
Neither reaction A nor reaction B releases energy.
Answers
Reaction B releases more energy than reaction A releases.The correct option is B)
The energy released by these reactions can be evaluated using the concept of nuclear binding energy. Nuclear binding energy is defined as the amount of energy required to break up a nucleus into its individual nucleons (protons and neutrons). This energy is a measure of the strong nuclear force that holds a nucleus together.
Reaction A involves the formation of a sodium chloride molecule by the transfer of one electron from a sodium atom to a chlorine atom. The energy released in this reaction is due to the formation of ionic bonds between the positively charged sodium ion and negatively charged chloride ion.
The energy released in this reaction is relatively low compared to nuclear reactions.In contrast, reaction B involves the decay of an oxygen isotope to form a nitrogen isotope, releasing a large amount of energy. The decay of an isotope involves the spontaneous breakdown of its nucleus due to a variety of factors.
This process releases a large amount of energy in the form of radiation and kinetic energy of the decay products.The energy released by nuclear reactions is much larger than that released by chemical reactions, such as reaction A. Therefore, option B, Reaction B releases more energy than reaction A releases, is the best description of the energy released per gram by these reactions.
Option B
For more such questions on Reaction visit:
https://brainly.com/question/25769000
#SPJ8
Recently one month's production of sodium hydroxide in the US was 2.01 billion pounds. The density of sodium hydroxide is 2.130 glcm:. What is the volume in cubic kilometers that were produced?
Answers
The volume of sodium hydroxide produced is approximately 4.277 × 10⁻⁴ km³.
To calculate the volume of sodium hydroxide in cubic kilometers that were produced, we must first convert the density from grams per cubic centimeter to kilograms per cubic meter. Then, we can use the mass of sodium hydroxide produced to find its volume.
Here are the steps to solve the problem:
Step 1: Convert the density from grams per cubic centimeter to kilograms per cubic meter
The density of sodium hydroxide is given as 2.130 g/cm³. To convert this to kg/m³, we need to divide by 1,000 (since 1,000 g = 1 kg) and then multiply by 10³ (since 1 cm³ = 10⁻⁶ m³).
2.130 g/cm³ × (1 kg/1,000 g) × (1,000,000 cm³/1 m³) = 2,130 kg/m³
Therefore, the density of sodium hydroxide is 2,130 kg/m³.
Step 2: Use the mass of sodium hydroxide produced to find its volume
The mass of sodium hydroxide produced is given as 2.01 billion pounds, which we must convert to kilograms:
2.01 billion pounds × (0.4536 kg/1 pound) = 910,476,000 kg
Now we can find the volume of sodium hydroxide produced:
Volume = Mass/Density = 910,476,000 kg/2,130 kg/m³ = 427,714 m³
However, the answer is requested in cubic kilometers, so we must convert from cubic meters to cubic kilometers:
427,714 m³ × (1 km/1000 m)³ = 4.277 × 10⁻⁴ km³.
for such more questions on density
brainly.com/question/1354972
#SPJ11
You set up a radio transmitter that operates by moving electric charges through a wire in a north-south direction. If you turn the transmitter so that the charges move in a east-west direction, what property of the radio waves will change
Answers
When you turn the transmitter so that charges move in a east-west direction, the polarization of the resulting electromagnetic waves will change.
What is polarization?Polarization is the orientation of the electric field vector of an electromagnetic wave. In electromagnetic wave generated by north-south current in a wire, electric field vector will be oriented in an east-west direction. If the current in the wire is moving in east-west direction, the electric field vector of resulting electromagnetic wave will be oriented in a north-south direction.
So, if you change the direction of electric charges in the wire, polarization of the resulting electromagnetic waves will change.
To know more about polarization, refer
https://brainly.com/question/3092611
#SPJ1