Answers
Answer:
b) At equilibrium, equal amounts of products and reactants are present.
Explanation:
At equilibrium , the ratio of product of concentration of products and product of concentration of reactants is constant .
A + B ⇄ C + D
[C] [ D] / [ A ] [ B ] = Constant
So, the statement ( b ) is false .
All other statements are true .
Related Questions
Which of the following processes is exothermic? A.cooking an egg B. the chemical reaction in a "cold pack" often used to treat injuries a camp fire C. melting of ice D. None of the above is exothermic
Answers
Answer: none of the above
Explanation: all are endothermic
What is the best conclusion based on this data?

Answers
Blood is a primary location for energy storage.Fat molecules contain more energy containing bonds than
anyone here know about the law of assumption?
Answers
Answer:
uhhh not really i don't even know what that is LOL
The following initial rate data are for the reduction of nitric oxide with hydrogen: 2 NO + 2 H2N2 + 2 H2O Experiment [NO]o, M [H2]o, M Initial Rate, M s-1 1 0.167 0.210 8.38×10-3 2 0.334 0.210 3.35×10-2 3 0.167 0.420 1.68×10-2 4 0.334 0.420 6.70×10-2 Complete the rate law for this reaction in the box below. Use the form k[A]m[B]n , where '1' is understood for m or n and concentrations taken to the zero power do not appear. Don't enter 1 for m or n. Rate = From these data, the rate constant is M-2s-1.
Answers
The complete rate law should be like: Rate = k[NO][H2]^2
Rate = k[NO]^x[H2]^y, where x and y are the exponents for the concentration of nitric oxide (NO) and hydrogen (H2) respectively.
The rate law can be determined by performing experiments with different initial concentrations of NO and H2 and observing how the initial rate changes.
From the data provided,
It can be seen that increasing the concentration of NO by a factor of 2 results in an increase in the initial rate by a factor of 2^(x).
Similarly, increasing the concentration of H2 by a factor of 2 results in an increase in the initial rate by a factor of 2^(y).
Based on this information, x = 1 and y = 2 can be determined, giving us the rate law:
Rate = k[NO][H2]^2
For more questions on rate law
https://brainly.com/question/1893345
#SPJ4
If the student’s estimate of the balloon’s volume was incorrect and the actual volume was 620 ml, would the amount of glucose that actually reacted be more than or less than the amount calculated in part (c)? Explain your response.
( C answer ) only 1.9 g of glucose reacted and only .0211 mol of co2 was formed.

Answers
The number of moles of CO2 produced is 0.021 moles
If the estimated volume of the balloon is wrong then the amount of glucose reacted must be more than is stated.
What is respiration equation?The respiration equation represents the chemical process of aerobic cellular respiration, which occurs in the mitochondria of cells and is the primary way in which cells generate energy in the form of ATP (adenosine triphosphate).
The equation of the reaction is;
C6H12O6 + 6O2 → 6CO2 + 6H2O + ATP
We know that;
Number of moles of glucose = 10 g/180 g/mol
= 0.056 moles
PV = nRT
n = PV/RT
n = 1 * 0.55/318 * 0.082
n = 0.021
Learn more about glucose:https://brainly.com/question/2252123
#SPJ1
The table below shows the average summer temperature increases for regions in Canada. Which of the following conclusions is supported by the data?
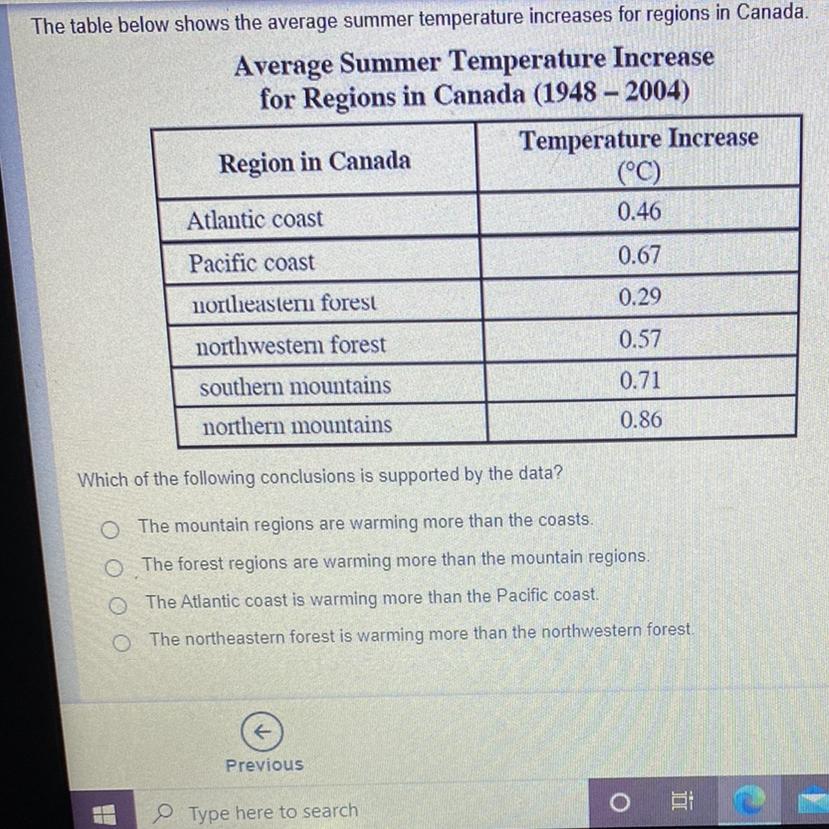
Answers
Answer:
The mountain regions are warming more than the coast
Explanation:
The average rise in temperature was the greatest in the mountains, and the coast average was less than the mountain average.
_______-the phase where the
chromosomes pull apart
Answers
Answer:
The answer to your question is ⇔ anaphase
So it would be anaphase is the phase where the chromosones pull apart.
Explanation:
The sister chromatids are pairs of identical copies of DNA joined at a point called the centromere. During anaphase, each pair of chromosomes is separated into two identical, independent chromosomes. The chromosomes are separated by a structure called the mitotic spindle.
I hope this helps and have a wonderful day!
Which statements are true of the electron cloud model? Check all that apply.
Answers
What is the difference between the R.M.M. and the molar mass of a compound?
Answers
Answer:
rmm doesn't need to include unit and molar mass need to include unit
chemist wished to find the volume of a large barrel and had no equipment for measuring the amount of water needed to fit it. He added of 400g of pure sodium chloride barrel and filled with chlorine free water. After thorough mixing a 100 ml sample of the solution re
Answers
Answer:
Place the object in the graduated cylinder, and record the resulting water volume as "b." Subtract the volume of the water alone from the volume of the water plus the object. For instance, if "b" was 50 milliliters and "a" was 25 milliliters, the volume of the irregularly shaped object would be 25 milliliters.
glucose is a six carbon sugar. Albumin is a protein with 607 amino acids. the average molecular weight of a single amino acid is 135 g/mol. there is no reason to run these solutes at the 20 MWCO because
Answers
There is no reason to run these solutes at the 20 MWCO because they are both much smaller than the MWCO of the membrane.
The MWCO (molecular weight cut off) is the molecular weight of a solute at which it will be retained by a membrane during a process such as ultrafiltration or dialysis. If a solute has a molecular weight higher than the MWCO of a membrane, it will be retained and not pass through the membrane. If the molecular weight of a solute is lower than the MWCO, it will pass through the membrane.
In this case, glucose has a molecular weight of 180 g/mol (6 carbons x 12 g/mol per carbon + 6 oxygens x 16 g/mol per oxygen) and albumin has a molecular weight of approximately 81,942 g/mol (607 amino acids x 135 g/mol per amino acid). Both of these solutes have molecular weights that are much lower than 20,000 g/mol, which is a typical MWCO for ultrafiltration or dialysis membranes.
They would both easily pass through the membrane and be lost during the process. Instead, a membrane with a much lower MWCO would be needed if we wanted to retain these solutes during a process such as ultrafiltration or dialysis.
Learn more about glucose here:
https://brainly.com/question/2396657
#SPJ1
describe some acidic oxides that can be prepared by the thermal decomposition of nitrates and carbonates.
Answers
Answer:
nitrogen dioxide and oxygen gas
Explanation:
the compounds are white solids and brown nitrogen dioxide and oxygen gases are also given off when heated.
using the periodic table which of the following is the correct electron configuration of vanadium in its ground state?

Answers
Answer:
Explanation:
The ground state electron configuration of ground state gaseous neutral vanadium is [Ar]. 3d3. 4s2 and the term symbol is 4F3/2.
The electronic configuration of the vanadium in its ground state is [Ar]4s²3d³. Therefore, option (A) is correct.
What is an electronic configuration?The electron configuration can explain how electrons are distributed in several energy levels of an atom of a chemical element. The number of electrons is written as a superscript of electron-containing atomic subshells in the electron configuration of an atom. For example, the electron configuration of nitrogen can be written as 1s²2s²2p³.
The principal quantum number (n) gives information about the maximum number of electrons that can occupy one electron shell. It is calculated from 2n², where ‘n’ is the principal quantum number.
The atomic number of the vanadium atom is 23 so it has 23 electrons to fill. Therefore, the electronic configuration of vanadium is [Ar]4s²3d³ as the energy of 4s orbital is less than the 3d orbital therefore, 4s orbital will be filled first then 3d orbital.
Learn more about electronic configuration, here:
brainly.com/question/5624100
#SPJ2
What a good example of hypothesis
Answers
Answer:
If you turn the lights on and off rapidly, then the bulb will burn out faster. It's some sort of prediction
Answer:
1. If I replace the battery in my car, then my car will get better gas mileage.
2. If I eat more vegetables, then I will lose weight faster.
3. If I add fertilizer to my garden, then my plants will grow faster.
4. If I brush my teeth every day, then I will not develop cavities.
5. If I take my vitamins every day, then I will not feel tired.
6. If 50 mL of water are added to my plants each day and they grow, then adding 100 mL of water each day will make them grow even more.
Explanation:
If a system receives 57 J of energy as heat and does 12 J of work on the surroundings, what is the change in the internal energy of the system?
Keep in mind the sign (+,-)
Answers
The change in the internal energy of the system is +69 J, indicating that the system has gained energy.
The change in internal energy of the system can be calculated using the First Law of Thermodynamics, which states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system on its surroundings.
In this case, the system receives 57 J of energy as heat, which means that the heat is added to the system, and the value is positive (+57 J). On the other hand, the system does 12 J of work on the surroundings, which means that the work is done by the system, and the value is negative (-12 J).
Using the First Law of Thermodynamics, we can calculate the change in internal energy of the system as:
ΔU = Q - W
ΔU = (+57 J) - (-12 J)
ΔU = 69 J
Therefore, the change in the internal energy of the system is +69 J, indicating that the system has gained energy.
To know more about First Law of Thermodynamics, visit:
https://brainly.com/question/3808473
#SPJ1
Draw a simple transverse wave and label the wavelength.draw it on a piece of paper
Answers
Answer:
I need this too but nobody knows how :(
Explanation:
Identify the double displacement reaction
2NH3+H2SO4
Answers
Answer:
......................
nh42so4
Put the following in order from SMALLEST to LARGEST: cell, nucleus, gene,
chromosome, DNA, organism,
Answers
Answer:
Here is the correct order (ascending order):
gene, DNA, chromosome, nucleus, cell, organism.
A gene is a segment of DNA that contains the instructions for building a protein or RNA molecule. DNA is the molecule that carries genetic information and makes up genes. A chromosome is a structure made of DNA and protein that carries genes. A nucleus is a membrane-bound organelle that contains chromosomes. A cell is the smallest structural and functional unit of living organisms, consisting of a nucleus (or nucleoid in prokaryotes) and cytoplasm. An organism is an individual living entity, such as a plant, animal, or bacterium, that consists of multiple cells.
At constant current is passed through an electrolytic cell containing molten MgCl2 for 18 hr. if 4.8 x 105 g of Cl2
are obtained. Calculate the current in Amperes.
Answers
The current passing through the electrolytic cell is approximately 2.02 x 10^4 Amperes.
To calculate the current in amperes, we need to use Faraday's laws of electrolysis and the stoichiometry of the reaction.
Faraday's laws state that the amount of substance produced or consumed during electrolysis is directly proportional to the quantity of electricity passed through the cell. The relationship is given by:
Q = nF
Where Q is the electric charge in coulombs (C), n is the number of moles of substance involved in the reaction, and F is Faraday's constant, which is equal to 96,485 C/mol.
In this case, the substance being produced is Cl2, and we know the mass of Cl2 produced, which is 4.8 x 10^5 g.
First, we need to calculate the number of moles of Cl2 produced:
Molar mass of Cl2 = 35.45 g/mol
Moles of Cl2 = mass / molar mass = (4.8 x 10^5 g) / (35.45 g/mol) ≈ 1.354 x 10^4 mol
Now we can calculate the quantity of electricity passed through the cell using Faraday's laws:
Q = nF
Q = (\(1.354 x 10^4\)mol) * (96,485 C/mol)
Q ≈ 1.308 x 10^9 C
The quantity of electricity is given in coulombs. To find the current, we need to divide this value by the time in seconds.
Given that the time is 18 hours, we convert it to seconds:
Time = 18 hours * 60 minutes/hour * 60 seconds/minute
Time = 6.48 x 10^4 seconds
Finally, we can calculate the current:
Current (I) = Q / Time
I = (1.308 x 10^9 C) / (6.48 x 10^4 s)
I ≈ 2.02 x 10^4 Amperes
Therefore, the current passing through the electrolytic cell is approximately 2.02 x 10^4 Amperes.
for more such question on electrolytic visit
https://brainly.com/question/17089766
#SPJ8
Name the following molecules and identify the homologous series to which each one belongs:
CH3CH2CH2Br
Answers
The name of the molecule is 1-bromopropane.
It belongs to the halogenated alkanes homologous series.
Homologous seriesA homologous series is a family of organic compounds with similar chemical properties and the same functional group which differ one from the next by a CH2 group.
Examples of homologous series are the alkanes, alkanes, alkanols, and halogenated alkanes.
Halogenated alkanesHalogenated alkanes are a family of organic compounds which differ from the parent alkane by a substitution of one or more of hydrogen atoms with alkanes.
Example is bromopropane, CH3CH2CH2Br; chloroethane, CH3Cl.
Therefore, the name of the molecule is 1-bromopropane. It belongs to the halogenated alkanes homologous series.
Learn more about homologous series at: https://brainly.com/question/17372951
If you picked up a hot coffee mug with lots of thermal energy using your hand, where will the thermal energy transfer to?
Answers
Answer:
It will go to your hand.
Explanation: If you touch a hot cup/mug you'll most likely burn yourself, so it would probably go to your hand.
7) How many molecules of CO2 are in 2.5 L at STP?
Answers
By using the ideal gas law and Avogadro's number, we find that there are approximately 6.72 × 10^22 molecules of CO2 in 2.5 L at STP.
To determine the number of molecules of CO2 in 2.5 L at STP (Standard Temperature and Pressure), we can use the ideal gas law and Avogadro's number.
Avogadro's number (N_A) is a fundamental constant representing the number of particles (atoms, molecules, ions) in one mole of substance. Its value is approximately 6.022 × 10^23 particles/mol.
STP conditions are defined as a temperature of 273.15 K (0 °C) and a pressure of 1 atmosphere (1 atm).
First, we need to convert the volume from liters to moles of CO2. To do this, we use the ideal gas law equation:
PV = nRT,
where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
Since we have STP conditions, we can substitute the values:
(1 atm) × (2.5 L) = n × (0.0821 L·atm/(mol·K)) × (273.15 K).
Simplifying the equation:
2.5 = n × 22.4149.
Solving for n (the number of moles):
n = 2.5 / 22.4149 ≈ 0.1116 moles.
Next, we can calculate the number of molecules using Avogadro's number:
Number of molecules = n × N_A.
Number of molecules = 0.1116 moles × (6.022 × 10^23 particles/mol).
Number of molecules ≈ 6.72 × 10^22 molecules.
Therefore, there are approximately 6.72 × 10^22 molecules of CO2 in 2.5 L at STP.
For more such questions on ideal gas law visit:
https://brainly.com/question/27870704
#SPJ8
Name the intermolecular force that corresponds to: an attraction between a partially positive region in one molecule and a partially negative region in another molecule. an attraction between two temporarily polarized molecules. an attraction between a negatively charged particle and a partially positive region in a molecule. an attraction between a partially positive hydrogen atom in a molecule and a partially negative and highly electronegative atom on another molecule.
Answers
Answer:
an attraction between a partially positive region in one molecule and a partially negative region in another molecule....dipole-dipole interaction
an attraction between two temporarily polarized molecules...dispersion forces
an attraction between a negatively charged particle and a partially positive region in a molecule....ion dipole interaction
an attraction between a partially positive hydrogen atom in a molecule and a partially negative and highly electronegative atom on another molecule....Hydrogen bonding
Explanation:
Some molecules have permanent dipole. As a result of this, the positive part of one molecule may attract the negative part of the other molecule leading to dipole-dipole interaction.
Dispersion forces occur in all molecules and is as a result of temporary polarization of a molecule due to instantaneous dipole–induced dipole attractions.
If a charged particle is attracted by a dipole in a molecule, we call it ion-dipole interaction.
When hydrogen is bonded to a highly electronegative element, the positive end of the dipole is on hydrogen while the negative end of the dipole is on the electronegative element. Molecular associations often result from this permanent dipole and is called hydrogen bonding, e.g, HF.
A given reaction has an energy difference between reactants and products (ΔH) of -23.7 kJ/mol, and and a forward activation energy (AE) of 27.9 kJ/mol. Which of the following are possible values of ΔH and AE for the forward reaction in the presence of a catalyst? Select all that apply.
Group of answer choices
ΔH = -12.1 kJ/mol and AE = 10.3 kJ/mol
ΔH = -43.9 kJ/mol and AE = 50.4 kJ/mol
ΔH = -23.7 kJ/mol and AE = 10.3 kJ/mol
ΔH = -9.27 kJ/mol and AE = 50.4 kJ/mol
ΔH = -23.7 kJ/mol and AE = 99.1 kJ/mol
ΔH = -23.7 kJ/mol and AE = 21.2 kJ/mol
Answers
The correct answer is option c ΔH = -23.7 kJ/mol and AE = 10.3 kJ/mol
What is catalyst?
A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy required for the reaction to occur, without undergoing any permanent chemical change itself. Catalysts work by providing an alternative pathway for the reaction that has a lower activation energy, allowing more reactant molecules to participate in the reaction and increasing the rate of product formation. Catalysts are widely used in industrial processes to increase the efficiency of chemical reactions and reduce the amount of energy required to produce a given amount of product. Some common examples of catalysts include enzymes in biological systems, transition metals such as platinum and palladium used in catalytic converters in cars, and acid or base catalysts used in the production of many chemicals.
Due to this, The activation energy (AE) is the minimum energy required to initiate a chemical reaction, and a catalyst can lower the activation energy required for a reaction to occur. However, a catalyst does not change the overall energy difference between the reactants and products (ΔH) of the reaction. Therefore, the only possible value for ΔH in the presence of a catalyst is still -23.7 kJ/mol.
Of the given answer choices, only ΔH = -23.7 kJ/mol and AE = 10.3 kJ/mol is a possible combination . The activation energy can be lowered to any value less than the original value of 27.9 kJ/mol, but it cannot be increased. Therefore, only the lower value of AE = 10.3 kJ/mol is a possible value in the presence of a catalyst.
To know more about Chemical reactions visit :-https://brainly.com/question/25769000
#SPJ1
How many grams are in 0.990 mol of neon?
mass in grams:
Answers
Explanation:
Multiply the given moles times the molar mass of neon, 20.180 g/mol (periodic table).
Answer:
Multiply the supplied moles by 20.180 g/mol, the molar mass of neon (periodic table).
Explanation:
Which of the following is considered a standard unit of length in the United States?
O square inch
O acre
O cubic yard
O yard

Answers
Answer:
Yard . I hope this helped:))
A Erlenmeyer flask is filled half full of water. A septum is added to seal the opening of the flask. Using a syringe, a soluble gaseous solute is introduced through the septum and into the air above the solvent. The solution swirled and then allowed to equilibrate. Select the factors that will cause an increase in the gas's solubility. The volume of the gas above the solution decreases when solution is added with a syringe. Using a syringe, a gas other than the solute is added to the flask. The temperature of the flask is raised. A reaction occurs between the gas solute and the solvent. The volume of the gas above the solution is increased by removing solution with a syringe. X
Answers
The factors that will cause an increase in the gas's solubility are:
1) The volume of the gas above the solution decreases when solution is added with a syringe.
This will increase the pressure of the gas above the solution, causing more gas molecules to dissolve into the solvent.
2) The temperature of the flask is raised.
An increase in temperature will cause the gas molecules to move faster and collide more frequently with the solvent molecules, increasing the solubility of the gas.
3) A reaction occurs between the gas solute and the solvent.
If the gas solute reacts with the solvent to form a new compound, the solubility of the gas will increase as the reaction will remove gas molecules from the gas phase and into the solution.
The other two factors (using a syringe to add a gas other than the solute and increasing the volume of the gas above the solution by removing solution with a syringe) will not cause an increase in the gas's solubility. Adding a gas other than the solute will not affect the solubility of the original gas solute, and increasing the volume of the gas above the solution will decrease the pressure of the gas, causing less gas molecules to dissolve into the solvent.
More questions on gas solubility can be found here: https://brainly.com/question/17295714
#SPJ11
Explain why this crossing could result in white hedgehogs even though brown is dominant
Answers
Answer:
Because The white gene is the recessive gene and the brown gene is the dominant gene hence all the offspring being brown instead of white :)
Explanation:
Thank me later!
Help me please.
This is Organic Chemistry

Answers
The condensed structural formulas as a bond-line formula are given in the attachment:
1. (CH3)2CHCH2CH3
2. CH2=C(CH2CH3)2
3. CH3CHCICH2CH(CH3)2
What is a bond-line formula?A bond-line formula, also known as a line-angle formula or skeletal formula, is a shorthand representation of a molecule in which the carbon and hydrogen atoms are implied and only the bonds between the atoms are shown.
In this representation, each vertex or endpoint of a line represents a carbon atom, and each line segment represents a bond between two adjacent atoms. The hydrogen atoms are not shown explicitly, but are assumed to be attached to the carbon atoms to satisfy the valence requirements.
Learn more about bond-line formula at: https://brainly.com/question/28454387
#SPJ1

Which equation shows an increase in entropy?
Hint: Look at the states of matter, g s l, of the chemicals in each equation. A C2H4(g) + H2(g) + C2H6(g) в Caco3(9) + Cao(s) - CO2(g) c Fe(s) + S (s) -+ FeS (s)

Answers
The equation C2H4(g) + H2(g) + C2H6(g) → Caco3(s) + Cao(s) + CO2(g) shows an increase in entropy due to the formation of a gas as a product. Option A
In this equation, the reactants on the left-hand side consist of gases (C2H4 and H2), while the products on the right-hand side include a solid (Caco3) and a gas (CO2).
When a reaction involves a change from gaseous to solid or liquid states, there is typically a decrease in entropy because the particles become more ordered and constrained in the solid or liquid phase.
Conversely, when a reaction involves the formation of gases, there is generally an increase in entropy because gases have higher degrees of molecular motion and greater freedom of movement compared to solids or liquids.
In the given equation, the reactants include three gaseous compounds (C2H4, H2, and C2H6), and one of the products is a gas (CO2). Therefore, the overall entropy of the system increases during this reaction.
The equation Fe(s) + S(s) → FeS(s) does not show an increase in entropy. Both the reactants (Fe and S) and the product (FeS) are solids. Since solids have lower entropy compared to gases or liquids, the entropy of the system does not increase in this reaction. Option A
For more such questions on entropy visit:
https://brainly.com/question/30481619
#SPJ8