Which statement describes how the types of radiation in the electromagnetic spectrum are different?
They have different amplitudes.
They have different wavelengths.
They travel at different speeds through space.
They travel different distances through space.
Answers
Answer:
they have different wavelengths
Related Questions
How is entropy related to the spontaneity of a reaction? AP3X
Answers
Answer:
S>0 contributes to spontaneity.
Explanation:
i just took the test on a pex :)
According to the law of thermodynamics, the relationship between entropy and the spontaneity of a reaction is S > 0.
What is entropy?Entropy is the total amount of energy in a closed system that is present there, but there are no uses for that energy. It is the unused energy for any work.
By the second law of thermodynamics, any spontaneous process results in an increase in the universe's overall entropy. The total of the entropy produced by the spontaneous process and the change in energy brought on by the heat flow is known as the net change in entropy of the system or S.
The reaction will be spontaneous if the delta G is negative. Delta G is the Gibbs free energy. So the entropy S will be greater that the reaction.
The Gibbs free energy equation is:
△G = △H − T△S
Thus, the relation is S > 0.
To learn more about entropy, refer to the link:
https://brainly.com/question/13386678
#SPJ2
What is the name of Br6F10 ?
Answers
Answer:
isopropyl alcohol
Explanation:
HOPE IT HELPS YOU
halogens are not the only atoms or groups that can be replaced by nucleophilic aromatic substitution as the following reaction shows. h512p601 what is the leaving group in this reaction? (formal charges have been intentionally omitted.) h512p60a h512p60c h512p60b
Answers
NO2 exists as O=N+-O, as a nucleophilic leaving group it should be in form of O=N-O¯. Hence, (a) is correct
What is nucleophilic aromatic substitution?
It is a reaction in in which a nucleophile displaces a leaving group on an aromatic ring.
The presence of EWG increases the rate of nucleophilic aromatic substitution
Nucleophilic aromatic substitution does not follow SN2 reaction mechanism, as the substitution takes place at trigonal carbon atom having sp3 hybridization.
It does not proceeds by SN2 reaction mechanism because of the steric hindrance of the benzene ring.
In the presence of a superior leaving group, the SN1 reaction pathway may be followed by nucleophilic aromatic substitution.
The SN1 reaction would result in the production of an aryl carbocation and the removal of the leaving group.
In most cases, elimination addition or addition elimination route is followed by nucleophilic aromatic substitution.
NO2 exists in the form of O=N+-O, as a nucleophilic leaving group it should be in form of O=N-O¯.
Learn more about Nucleophilic substitution reaction at :
brainly.com/question/24316838
#SPJ4
iron is used to make utensils.which properties of iron enable it to make utensil possible?
Answers
Answer:
Good heat conductivity, low chemical reactivity, strength and durability are the qualities of Iron that make it a good material for making utensils.
Explanation:
Some materials are great in some areas but not so good in others. Iron is a great compromise. It has very good heat transfer for nice even heating and is tough.
Calculate the number of carbon atoms in a 140.0g sample of glucose (C6H12O6) . Be sure your answer has a unit symbol if necessary, and round it to significant digits.
Answers
Answer:
There are 2.81 * 10^24 atoms present
Explanation:
To get this, we need to know the number of moles present in 140gramms of carbon.
Mathematically;
number of moles = mass/molar mass
molar mass of glucose is = 180 g/mol
So the number of moles will be ;
140/180 = 0.7778 mole
1 mole of glucose contains 6 moles of carbon;
so 0.7778 moles will contain = 4.667 moles
Mathematically, the number of atoms in 1 mole is 6.02 * 10^23 atoms
The number of atoms in 4.667 moles will be :
4.667 * 6.02 * 10^23 = 2.81 * 10^24 atoms
There is an average of ______ inches of rainfall a year.
50-65 inches
20-35 inches
10-20 inches
35-50 inches
Answers
The average rainfall varies from place to place. However, the average rainfall is about 10-20 inches.
What is rainfall?Rain fall refers to a situation in which the water that is found at the surface of the earth is evaporated, condenses in the atmosphere and then falls again to the surface of the earth as precipitation or rainfall. It is an essential component of the water cycle.
We know that the average rainfall varies from place to place. However, the average rainfall is about 10-20 inches.
Learmn more about rainfall: https://brainly.com/question/11739819?
A beaker with 155 mL of an acetic acid buffer with a pH of 5.000 is sitting on a benchtop. The total molarity of acid and conjugate base in this buffer is 0.100 M. A student adds 6.70 mL of a 0.360 M HCl solution to the beaker. How much will the pH change?
Answers
If the total molarity of acid and conjugate base in the buffer is 0.100 M and then adds 6.70 mL of a 0.360 M HCl solution is to the beaker, the pH of the buffer solution changes from 5.000 to 4.71 after the addition of 6.70 mL of 0.360 M HCl solution and the pH decreases by 0.290.
To calculate the change in pH of the buffer solution after the addition of the given volume of HCl, we can use the Henderson-Hasselbalch equation which is given as;
pH = pKa + log [A⁻] / [HA]
where, [A⁻] is the molar concentration of the conjugate base, [HA] is the molar concentration of the weak acid, and pKa is the dissociation constant of the weak acid.
In the given buffer solution, acetic acid is the weak acid and the acetate ion is the conjugate base. The dissociation constant of acetic acid is given as;
Ka = 1.8 × 10⁻⁵
Therefore, the pKa of acetic acid is given as;
pKa = - log Ka
= - log (1.8 × 10⁻⁵)
= 4.74
The buffer solution is 0.100 M. Therefore, the molar concentration of acetate ion and acetic acid in the buffer solution is 0.050 M respectively. This is because the buffer solution contains equal amount of weak acid and conjugate base.We are also given that 6.70 mL of 0.360 M HCl is added to the buffer solution. The number of moles of HCl added is given as follows;
moles of HCl = concentration × volume
= 0.360 × 6.70 / 1000
= 0.002412 mol
Since acetic acid is the weaker acid, the added H⁺ ions will react with acetate ions to form acetic acid. The number of moles of acetate ion and acetic acid after the addition of HCl is given as follows;
moles of acetate ion = moles of initial acetate ion - moles of HCl added
= 0.050 - 0.002412
= 0.04759 mol
moles of acetic acid = moles of initial acetic acid + moles of HCl added
= 0.050 + 0.002412
= 0.05241 mol
Using the new molar concentrations, we can calculate the new pH of the buffer solution as follows;
pH = pKa + log [A⁻] / [HA]
= 4.74 + log (0.04759 / 0.05241)
= 4.74 - 0.034
= 4.71
Learn more about change in pH: https://brainly.com/question/30969699
#SPJ11
How does a nuclear fusion work. List the elements involved
Answers
Answer: 1. Two protons within the Sun fuse. Most of the time the pair breaks apart again, but sometimes one of the protons transforms into 2.a neutron via a weak nuclear force. Along with the transformation into a neutron, a positron, and neutrino are formed. This resulting proton-neutron pair that forms sometimes is known as deuterium.
3. A third proton collides with the formed deuterium. This collision results in the formation of a helium-3 nucleus and a gamma ray. These gamma rays work their way out from the core of the Sun and are released as sunlight.
4Two helium-3 nuclei collide, creating a helium-4 nucleus plus two extra protons that escape as two hydrogens. Technically, beryllium-6 nuclei form first but are unstable and thus disintegrate into the helium-4 nucleus.
Explanation:
The final helium-4 atom has less mass than the original 4 protons that came together (see E=mc2). Because of this, their combination results in an excess of energy being released in the form of heat and light that exits the Sun, given by the mass-energy equivalence. To exit the Sun, this energy must travel through many layers to the photosphere before it can emerge into space as sunlight. Since this proton-proton chain happens frequently - 9.2 x 1037 times per second - there is a significant release of energy.[3] Of all of the mass that undergoes this fusion process, only about 0.7% of it is turned into energy. Although this seems like a small amount of mass, this is equal to 4.26 million metric tonnes of matter being converted to energy per second.[3] Using the mass-energy equivalence, we find that these 4.26 million metric tonnes of matter are equal to about 3.8 x 1026 joules of energy released per second!
A gaseous mixture consisting of nitrogen, argon, and oxygen is in a 3.5-L vessel at 25C. Determine the number of moles of oxygen if the total pressure is 98.5kPa and the partial pressure of nitrogen and argon are 22.0kPa and 50.0kPa, respectively.
Answers
Answer:
Number of moles of oxygen = 0.037 mol
Explanation:
Given data:
Total pressure = 98.5 KPa
Partial pressure of nitrogen = 22.0 KPa
Partial pressure of argon = 50.0 KPa
Volume = 3.5 L
Temperature = 25°C (25+273= 298K)
Number of moles of oxygen = ?
Solution:
Total pressure = P(N₂) + P(O₂) + P(Ar)
98.5 KPa = 22.0 KPa +P(O₂) + 50.0 KPa
98.5 KPa = 72.0 KPa +P(O₂)
P(O₂) = 98.5 KPa - 72.0 KPa
P(O₂) = 26.5 KPa
KPa to atm:
26.5 KPa/ 101 = 0.262 atm
Number of moles of oxygen:
PV = nRT
n = PV/RT
n = 0.262 atm × 3.5 L / 0.0821 atm.L/mol.K × 298 K
n = 0.917atm.L /24.47atm.L/ mol
n = 0.037 mol
The solute will dissolve quicker if the solute is more?
A. shaken.
B. saturated.
C. cooled.
D. settled.
Answers
Answer:
A) Shaken is the correct answer
Explanation:
The other three don't fit in with the solution if you think about it. When you shake something with lemonade crystals (Like a lemonade flavor packet in poured in a water bottle) it dissolves faster when you shake it rather than it sit on the bottom for 15 minutes! :) .
Hope this helps! :)
Answer:
The Person above me is correct
Explanation:
I got it right first try 100%
what is the molecular weight of a gas which diffuses 1/50 as fast as hydrogen
Answers
Silver has the density of 9.32 g/cm^3. Find the length of one side of a 184 g silver cube. Assume now that the silver cube above was melted and reshaped into a sphere. Find the sphere’s diameter
Answers
d = 3.35cm
c3h6 has a double bond in its carbon skeleton? a. true b. false
Answers
\(C_3H_6\) has a double bond in its carbon skeleton. This is a true statement.
Carbon skeleton refers to the chain of carbon atoms that make up an organic molecule. The presence or absence of double bonds in the carbon skeleton affects the properties of the molecule and how it interacts with other molecules. In \(C_3H_6\), there are three carbon atoms arranged in a linear chain, with each carbon atom forming single covalent bonds with two hydrogen atoms. The remaining valence electrons on each carbon atom form a double bond between the first and second carbon atoms.
This double bond is responsible for the unsaturated nature of the molecule. \(C_3H_6\)is an example of a simple alkene, also known as propene. Its carbon skeleton and double bond make it a versatile molecule that can be used in various applications, including the production of plastics, rubber, and other materials.
Learn more about carbon skeleton from this link:
https://brainly.com/question/3707087
#SPJ11

Nickel has a cubic unit cell. The edge of the unit cell is 3.524
x 10^(-8)cm. Determine the atomic radius of Nickel.
Answers
The approximate atomic radius of nickel is 1.532 × 10^(-8) cm.
In a cubic unit cell, the body diagonal length (diagonal that passes through the center of the unit cell) is equal to four times the atomic radius (4r). We can use this relationship to find the atomic radius of nickel.
Given: Edge length of the unit cell (a) = 3.524 × 10^(-8) cm
The body diagonal length is given by:
Diagonal length (d) = a√3
Substituting the given values:
d = (3.524 × 10^(-8) cm) × √3
Now, we can calculate the atomic radius (r) by dividing the diagonal length by 4:
r = d / 4
Performing the calculations:
r = [(3.524 × 10^(-8) cm) × √3] / 4
r ≈ (3.524 × 10^(-8) cm) × (1.732 / 4)
r ≈ 1.532 × 10^(-8) cm
Therefore, the approximate atomic radius of nickel is 1.532 × 10^(-8) cm.
Know more about atomic radius here
https://brainly.com/question/18095927#
#SPJ11
How does the arrangement of atoms affect the classification of matter?
Answers
On the basis of arrangement of atoms, the matter is classified into three states: solid, liquid and gas.
In Chemistry, there are three fundamental states of matter based on the arrangement of molecules and atoms inside it.
Generally, solids have strict and rigid arrangement of atoms as they posses strong intermolecular forces and have high melting and boiling points. solids have fixed composition of atoms.
Similarly, liquids have loose arrangement of atoms and molecules keep moving randomly in a liquid. Liquids have low melting and boiling points as compared to solids. Gases have weakest intermolecular forces and atoms are arranged randomly in them. Also, their molecules keep moving in all directions.
If you need to learn more about atoms click here:
https://brainly.com/question/6258301
#SPJ4
What is thermal equilibrium?
Answers
31. Can two (or more) types of matter occupy the same space at the same time?
32. What is hardest state of matter?
33. What is the softest state of matter?
Answers
32. Solid state
33. Liquid state
mse 2001 compared to a semi-crystalline polymer of the same composition, a completely amorphous polymer is expected to:
Answers
The semi-crystalline polymer of the same composition, a completely amorphous polymer is expected to the crystallites scatter light.
Crystallization of polymers is a manner associated with the partial alignment of their molecular chains. these chains fold together and shape ordered areas called lamellae, which compose larger spheroidal systems named spherulites.
Semi-crystalline plastics can be taken into consideration for a selection of applications. choosing a plastic material for use in excessive temperature surroundings calls for a cautious assessment of fabric residence information. View our interactive Thermoplastics Triangle to evaluate substances.
Polyethylene is a partially crystalline solid whose residences are exceedingly dependent on the relative content of the crystalline section and amorphous section, i.e., crystallinity. Polyethylene is a polymer polymerized from monomeric ethylene.
Learn more about semi-crystalline polymer here:-https://brainly.com/question/28383719
#SPJ4
After 1 kg of U-235 undergoes fission, the mass of the products is 8.4 x 10^-4 kg less than the initial 1 kg. How much energy was produced by the fission of 1 kg of U-235?
Use Einstein's equation, E = mc2, where E is energy in Joules, m is mass in kilograms, and c is the speed of light, 3 x 108 m/s.)
Answers
Answer:
Uranium-235 is a popular choice of fuel for nuclear reactors. Instead of allowing 1 kg of U-235 to decay naturally, imagine it is used as fuel in a nuclear > reactor. It is bombarded with neutrons, causing it all to fission in a matter of days. U-235 undergoes fission, the mass of the products is 8.4 x 10-4 kg
Explanation:
The first nuclear reactor to achieve controlled nuclear disintegration was built in the nuclear reactions presently used or studied by the nuclear power industry in nuclear fission a large nucleus is split into two medium-sized nuclei. About 5600 tons (5.1 X 106 kg) of coal are required to produce the same amount of.
someone help please i cant figure this out
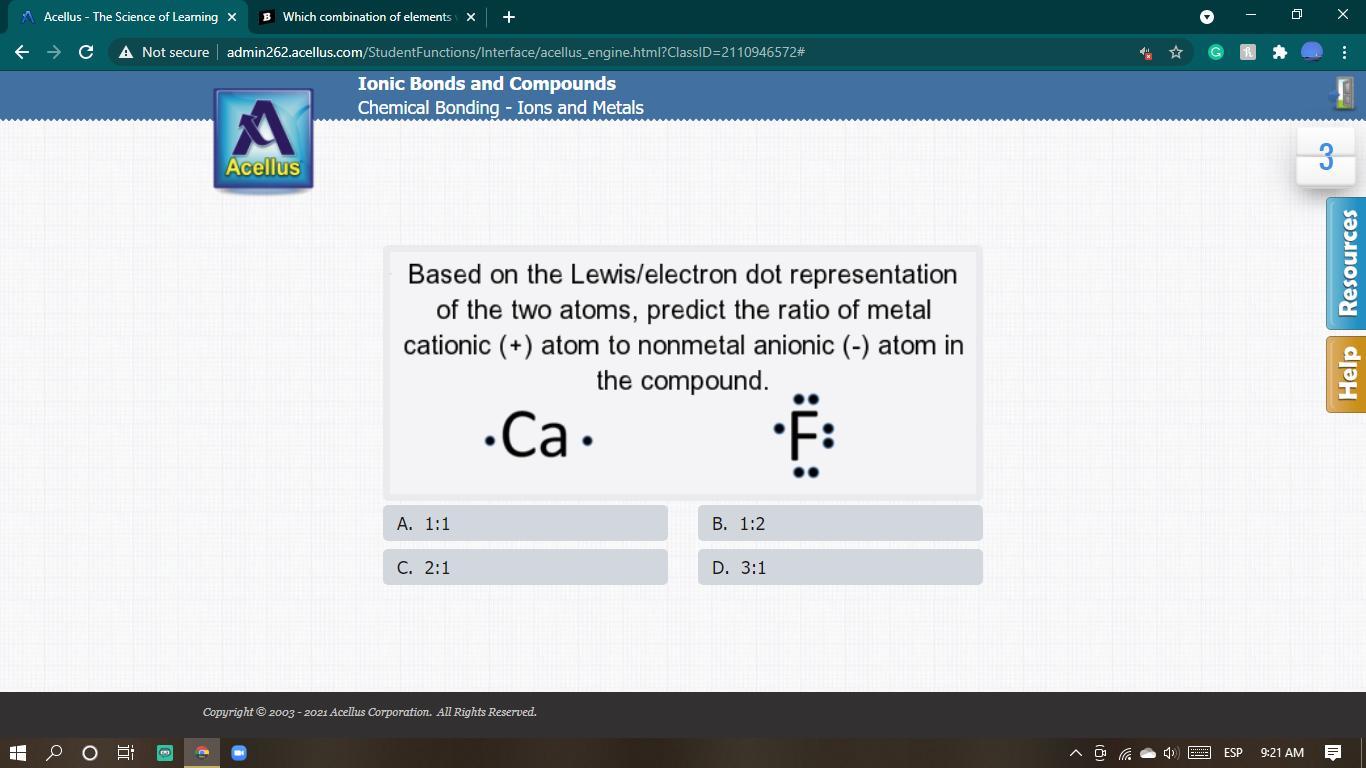
Answers
Answer: 2 : 1
Explanation:
Cation :
Ca - calcium = atomic number = 20
Electron dot configuration : 2, 8, 8, 2
Ca losses 2 electrons in its outermost shell and thus has a charge of 2+ in other to attain a stable octet state.
Anion:
F - has 7 valence electrons and thus needs 1 electron to achieve a stable octet state, hence it accepts one electron has has a charge of (-1)
Therefore,
Ratio of cation (+) to negative (-) = 2 :1
1. Mention the 4 methods of separation. Explain each method with diagrams and give suitable examples.
2. Define and give 2 examples for each of the above.
Answers
Using techniques like distillation, filtration, and chromatography, which employ variations in the components of a mixture's physical qualities to separate them, mixtures can be physically separated.
What is the separation approach, with an example?Filtration is the most used technique for separating a liquid from an insoluble material. Consider the combination of sand and water, for instance. Here, solid particles are eliminated from the liquid through filtration. Different filtering agents, such as filter paper or other materials, are frequently utilized.
What types of separation techniques are there?Filtration, hand-picking, threshing, winnowing, sieving, sedimentation and decantation, magnetic separation, centrifugation, evaporation, distillation, and other processes are a few of the mixture preparation techniques that are covered in detail in this article.
Learn more about distillation here:
https://brainly.com/question/13345735
#SPJ1
A solid metal sphere has a radius of 3.53 cm and a mass of 1.796 kg. What is the density of the metal in g/cm^3? (The volume of sphere is V = 4/3 pi r^3.) a) 34.4 g/cm^3 b) 0.103 g/cm^3 c) 121 g/cm^3 d) 9.75 g/cm^3
Answers
The density of the metal sphere is 9.75 g/cm³ (Option D).
To find the density of the metal sphere, we can use the formula for density, which is density = mass/volume. First, we need to find the volume of the sphere using the given formula V = 4/3 π r³, where r is the radius of the sphere. Then, we can convert the mass of the sphere to grams and use the formula to find the density.
Given radius (r) = 3.53 cm and mass = 1.796 kg.
1. Calculate the volume of the sphere:
V = (4/3) * π * (3.53)³
V ≈ 184.3 cm³
2. Convert the mass to grams:
1 kg = 1000 g
Mass = 1.796 kg * 1000
Mass = 1796 g
3. Calculate the density:
Density = Mass/Volume
Density = 1796 g / 184.3 cm³
Density ≈ 9.75 g/cm³
Therefore, the density of the metal in the sphere is approximately 9.75 g/cm³.
Know more about Density of metal here:
https://brainly.com/question/14599570
#SPJ11
Describe a method to separate the dyes in coloured inks. [4 marks]
A paper chromatogram from a mixture of two substances, A and B, was obtained using a solvent of propanone. Substance B was found to travel further up the paper than substance A.
What does this tell you about substances A and B. [1 mark]
Look at the boiling points of the three liquids in the table: Liquid Boiling point in °C water 100 ethanol 78 propanol 97 A mixture was made by stirring together equal volumes of these three miscible liquids. Evaluate the effectiveness of fractional distillation as a way of separating this mixture into the three pure liquids.
Answers
Chromatography is a method of separating out materials from a mixture.
Aim: To separate the dye present in ink by the process of evaporation.
Materials required: Beaker, watch glass, water, ink and stove.
Procedure: Take a beaker and fill it to half its volume with water. Keep 3, glass on the mouth of a beaker. Put few drops of ink on the watch glass. Heat the beaker and observe the watch glass.
Observations: We observe some fumes coming from the watch glass. Continue heating till you do not observe any further change on the watch glass. A small residue will be remained on the watch glass.
Inference: We know that ink is a mixture of a dye in water. The residue remained on the watch glass is the dye present in the ink.
Chromatography is a method of separating out materials from a mixture. Ink is a mixture of several dyes and therefore we can separate those colors from one another using chromatography.
Learn more about chromatography at
https://brainly.com/question/1558595
Laura has three beakers. Each contains 200 cm³ of a colourless liquid. Describe how Laura could find out which beakers contain pure water, and which contain solutions. Explain your answer.
Answers
Laura could use a few different methods to determine which beakers contain pure water and which contain solutions. One method is to test the boiling point of each liquid. Pure water boils at 100 degrees Celsius at standard pressure. If the liquid in a beaker boils at a temperature higher than 100 degrees Celsius, it is likely a solution and not pure water. Another method is to test the freezing point of each liquid. Pure water freezes at 0 degrees Celsius at standard pressure. If the liquid in a beaker freezes at a temperature other than 0 degrees Celsius, it is likely a solution and not pure water.
Another method is through density test. Pure water has a density of 1g/cm³ at 4°C. Laura can use a hydrometer, which is an instrument that measures the density of a liquid to check if the density of the liquids in the beakers is equal to 1g/cm³. If it is not, then it is not pure water.
Additionally, Laura could also test the conductivity of the liquids. Pure water is a poor conductor of electricity, whereas solutions can conduct electricity. Laura could use a conductivity meter to check the conductivity of the liquids. If a liquid conducts electricity, then it is likely a solution and not pure water.
Finally, Laura could also use a refractometer, which measures the refractive index of the liquid. The refractive index of pure water is 1.333 and any deviation from this value indicates the presence of dissolved solutes.
It's important to notice that no single test can confirm that a liquid is pure water, but a combination of tests can give us a strong indication of it.
explain the difference in behavior between water and the buffer with addition of acid or base. are your results as expected? why or why?
Answers
The adding of an acid or base to a buffer seems to have no effect on the pH of the buffer. In contrast, going to add acid or base to unbuffered water drastically changes the pH.
Any hydrogen-containing substance skilled of making a donation a proton (hydrogen ion) to that other substance is defined as an acid. A base is a compound or ion that really can accept an acid's hydrogen ion. The sour flavours of weak acids is generally described by the contaminant that emits hydrogen ions in water and formation salts by incorporating with these metals. Acids have a bitter aftertaste and cause certain dyes to turn red. buffer is an aqueous solution that withstands adjustments in pH upon on the addition of either an acid or a base". Furthermore, adding water to a buffer or going to allow it to evaporate from of the buffer has no significant effect just on pH of the buffer.
Learn more about ph here:
https://brainly.com/question/29766400
#SPJ4
()3C− − on reaction with HI gives () − − as
the main products and not () − and −
Answers
3C⁻⁻ on reaction with HI gives I⁻⁻⁻ as the main products and not H⁻ and C₂H₅I.
When 3C⁻⁻ is reacted with HI, the reaction product obtained is I⁻⁻⁻ as the main product. The C₂H₅I and H⁻ are not produced in significant quantities and cannot be considered the main product.The 3C⁻⁻ compound reacts with HI in the presence of a solvent to produce hydrogen gas, H⁻, C₂H₅I, and I⁻⁻⁻. The primary product obtained is I⁻⁻⁻ because it is stable and has a higher energy than C₂H₅I and H⁻.However, the reaction can be controlled to obtain C₂H₅I and H⁻ as the primary products by changing the reaction conditions. The reaction must be carried out in anhydrous conditions and at a low temperature so that the reaction proceeds in the desired direction.
3C⁻⁻ on reaction with HI gives I⁻⁻⁻ as the main products and not H⁻ and C₂H₅I. However, the reaction can be controlled to obtain C₂H₅I and H⁻ as the primary products by changing the reaction conditions.
To know more about hydrogen visit:
brainly.com/question/30623765
#SPJ11
what is the mass, in grams, of 2.4 x 10^23 atoms of Zn
Answers
Answer: 26.07 g Zn if need to correct sig figs its 26 g Zn
Explanation:
use stoichiometry to solve
2.4 X 10^23 atoms Zn X (1mole Zn / 6.02 X 10^23 atoms Zn) X (65.38 g Zn/ 1 mole Zn) =
26.07 g Zn
Which of the following processes is one of the early steps in the formation of sedimentary rock?
A) Weathering of existing rock
B) Crystallization of molten minerals
C) Melting of rock in volcanoes
D) Chemical reactions that produce heat
Answers
Answer:
weathering of existing rocks
which natural resources have most benefitted the economic development of central asian countries?
Answers
The extraction of oil and natural gases have benefitted most to the economic development of central Asian countries.
In the central Asia, the most significant resources business is the business of oil and natural gases. These natural gases are found in ample amount in these countries like Saudi Arab and Qatar.
Furthermore, the region possesses substantial mineral reserves, including as gold, silver, copper, and uranium, which contribute to export profits and job development. The combination of these natural resources has been critical in propelling regional economic growth and development. However, over-reliance on these resources has created problems such as environmental deterioration and economic volatility.
To know more about Oil and natural gas, visit,
https://brainly.com/question/2605522
#SPJ4
Please help, it’s due tonight and I can’t figure it out.

Answers
I don't know men sorry but You can see if some one have a idea about the