Answers
Answer:
B) water and salt is the result of neutralization reaction
Water and salts are the product of a neutralization reaction.
NEUTRALIZATION REACTION:
Neutralization reaction is a type of reaction between an acid and a base to form a salt and water. In a neutralization reaction, the hydrogen ion (H+) and hydroxyl ion (OH-) are released from the acid and base respectively and are combined to form water. An example is the reaction between hydrochloric acid (HCl) as an acid and sodium hydroxide (NaOH) as a base to form sodium chloride (NaCl) as salt and water (H2O). Therefore, water and salts are the product of a neutralization reaction.Learn more at: https://brainly.com/question/20038776?referrer=searchResults
Related Questions
a gas has an initial volume of 3,480 mL and an initial temperature of - 70.0 C. what must be the temperature of the gas in kelvin if its volume is reduced to 2,450 mL
Answers
The temperature of the gas in Kelvin, after its volume is reduced to 2,450 mL, is approximately 143.27 K.
To determine the temperature of the gas in Kelvin after its volume is reduced, we can use the combined gas law, which relates the initial and final conditions of pressure, volume, and temperature for a given amount of gas.
The combined gas law equation is:
(P₁ * V₁) / T₁ = (P₂ * V₂) / T₂
Where P₁ and P₂ are the initial and final pressures, V₁ and V₂ are the initial and final volumes, T₁ is the initial temperature in Kelvin, and T₂ is the final temperature in Kelvin.
Given that the initial volume V₁ is 3,480 mL, the initial temperature T₁ is -70.0 °C (which needs to be converted to Kelvin), and the final volume V₂ is 2,450 mL, we can substitute these values into the equation.
To convert -70.0 °C to Kelvin, we add 273.15 to it, resulting in T₁ = 203.15 K.
Now we can solve for T₂:
(T₂ * V₁) / T₁ = V₂
T₂ = (V₂ * T₁) / V₁ = (2,450 mL * 203.15 K) / 3,480 mL
Simplifying the equation, we find:
T₂ ≈ 143.27 K
Therefore, the temperature of the gas in Kelvin, after its volume is reduced to 2,450 mL, is approximately 143.27 K.
For more question on temperature
https://brainly.com/question/4735135
#SPJ8
Examine the substances listed. Identify the substance(s) that represents a single element.
SUBSTANCE: soil, copper pipe, water. CO2*
soil
copper pipe
water
CO2
Answers
Answer:
the answer is copper pipe
True or false let me know ASAP

Answers
Answer:
false
Explanation:
How do you get the formula of an ionic compound from the name of an ionic compound?
Ex:
Calcium chloride > CaCl2
Answers
To get the formula of an ionic compound from its name, we need to identify the ions present and balance their charges to determine the subscript of each ion.
Ionic compounds are composed of positively charged ions (cations) and negatively charged ions (anions). The name of an ionic compound provides information about the ions present in the compound.
In the case of calcium chloride, the name tells us that the compound contains a calcium ion (Ca²⁺) and a chloride ion (Cl⁻). To write the formula of the compound, we need to balance the charges of the ions. Since the calcium ion has a charge of +2 and the chloride ion has a charge of -1, we need two chloride ions to balance the charge of one calcium ion. Therefore, the formula of calcium chloride is CaCl₂.
To determine the formula of other ionic compounds from their names, we need to follow the same process of identifying the ions present and balancing their charges. For example, in sodium sulfide (Na₂S), the name tells us that the compound contains a sodium ion (Na⁺) and a sulfide ion (S²⁻). To balance the charges, we need two sodium ions for every one sulfide ion, so the formula of sodium sulfide is Na₂S.
To learn more about ionic compound, here
https://brainly.com/question/3222171
#SPJ1
Using the guideline for oxidation numbers, write the reduction half-reactions for the following:
• O
• P
• Cu
Answers
The reduction half-reactions for O, P, and Cu:
• O: O2 + 4 e- → 2 O2-
• P: HPO42- + 2 H+ + 2 e- → H3PO4
• Cu: Cu2+ + 2 e- → Cu+
To write the reduction half-reactions for O, P, and Cu, we need to determine the oxidation numbers for each element. The guidelines for assigning oxidation numbers are:
The oxidation number of an atom in its elemental form is 0.The oxidation number of a monatomic ion is equal to its charge.The sum of the oxidation numbers of all atoms in a neutral molecule must be 0.The sum of the oxidation numbers of all atoms in a polyatomic ion must be equal to the charge of the ion.Using these guidelines, we can determine the oxidation numbers for O, P, and Cu:
O: Oxygen is a diatomic molecule, so its oxidation number is 0 in O2.P: The most common oxidation state for phosphorus is +5 in its compounds, but it can also have oxidation states ranging from -3 to +5.Cu: The most common oxidation state for copper is +2, but it can also have oxidation states ranging from +1 to +4.To know more about the Reduction half-reaction, here
https://brainly.com/question/18403544
#SPJ4
How does an igneous or metamorphic rock become a sedimentary rock?
Answers
Answer: Usually, the rock pieces, called sediments, drop from the wind or water to make a layer. The layer can be buried under other layers of sediments. After a long time the sediments can be cemented together to make sedimentary rock. In this way, igneous rock can become sedimentary rock.
Explanation:
Why is scientific notation used?
to round numbers to the nearest whole number
to promote reproducibility of data
to increase the validity of data
to express very large or very small numbers
Answers
The correct option is (d) To express very large or very small number.
What is the Scientific Notation?
Scientific notation is a way to present numbers that are too large or too small to be easily written in decimal form. The three components of scientific notation are coefficient, base and exponent. The proper format to write a scientific notation is a x 10^b, where a is a number or decimal number and b is the power of 10 to make scientific notation equivalent to original number. When a number between 1 and 10 is multiplied by a power of 10 then the number is expressed in scientific notation. For example, 10000000 can be written as 10⁷, which is the scientific notation and the exponent is positive here. Similarly, for the negative exponent 0.000001 can be can be represented as 10-⁷. Hence, the scientific notation is used to express very large or very small numbers.
Learn more about Scientific Notation here: https://brainly.com/question/15361382
#SPJ1
An EpiPen, used to treat anaphalactic allergic reactions, contains 0.30 mg of epinephrine. The concentration of epinephrine in each syringe is 1.0 mg/mL. What is the volume, in milliliters (mL), of solution in each syringe
Answers
Answer:
0.3mL
Explanation:
Mass = 0.30mg
Concentration = 1.0 mg/mL
Volume = x
The relationship between the three parameters is given as;
Concentration = Mass / Volume
Making Volume our subject of interest we have;
Volume = Mass / Concentration
Substituting the values we have;
Volume = 0.30 mg / 1 mg/mL = 0.3mL
Calculate the number of moles of gas produced from the reaction of 2.00g of potassium with an excess amount of water.
Answers
The number of moles of gas produced from the reaction of 2.00g of potassium with an excess amount of water is 0.025 moles.
The reaction of potassium with an excess amount of water is:
2K + 2H\(_2\)O \(\rightarrow\) 2KOH + H\(_2\)
To calculate the moles of hydrogen gas first we need to calculate moles of potassium in 2.00g
No. of moles = (mass) / (molecular mass)
The mass given is 2.00 g and the Molecular mass is 39.09 units
∴ No. of moles = (2) / (39.09) = 0.05
From the above reaction, we get that 2 moles of potassium give 1 mole of hydrogen gas. Thus, 0.05 moles of potassium gives 0.025 moles of hydrogen gas.
Therefore, the no. of moles of hydrogen gas produced is 0.025 moles.
To learn more about potassium,
brainly.com/question/13321031
For a certain analysis a stock solution of 1000 mg/L iron is needed. The only iron salt that is present is iron(II) sulphate (FeSO4·7H2O). The mass percentage of iron in this salt is 20.1%. Calculate in mg how much iron(II) sulphate needs to be weighed out to make a 50.0 mL solution
Answers
According to percent solution, 100.5 mg iron(II) sulphate needs to be weighed out to make a 50.0 ml solution.
Percent solution is defined as a convenient way to record concentration of solution.It is a expression which relates solute to solvent as,mass of solute/mass of solution ×100.There are two types of percentage solutions percent weight by volume and percent volume by volume .Advantages of using percent solutions is that molecular weight of compound is not required.
It is calculated as, 20.1×50=100.5 g.
Learn more about percent solution,here:
https://brainly.com/question/4549662
#SPJ1
What is the function of a lyase enzyme?
a. To assist the substrate by binding to the enzyme, enabling substrate to active site engagement
b. To facilitate a reaction of one substrate to form two products with the use of water
c. To facilitate a reaction of one substrate to form two products without the use of water
d. To tell fibs
Answers
Answer:
c. To facilitate a reaction of one substrate to form two products without the use of water
Explanation:
A lyase is an enzyme that catalyzes - accelerates the chemical reaction - in which a substrate is broken into two molecules. The reaction does not involve hydrolysis or oxidation, so the water molecule is not included in the chemical reaction. Thus, the enzyme facilitates the reaction in which a molecule (substrate) is decomposed into two molecules with the elimination of chemical bonds.
How many mg does an 830 kg sample contain?
Answers
Answer:
A sample of 830 kg would contain 830000000 mg
Explanation:
Which family correctly represents the element in group 1 on the periodic table
Answers
Answer:
Alkali Metals
Explanation:
I used Google
Answer:
Alkali metals
Explanation:
How much work (in J ) is involved in a chemical reaction if the volume decreases from 4.40 to 1.39 L against a constant pressure of 0.872 atm?
Answers
The work involved in a chemical reaction is -2.62J
What is work done in a chemical reaction?Work is done in the chemical reaction when gases expand and compress against a constant external pressure.
The work done by a gas is given by the formula;
w = pΔv
p = pressure of the gas
Δv = change in the volume of the gas
When the gas does work the volume of a gas increases (ΔV>0) and the work done is negative.When work is done on the gas, the volume of the gas decreases (ΔV<0) and work is positive.Given,
Pressure = 0.872 atm
Initial volume = 4.40 L
Final volume = 1.39 L
Work done = Pressure ( Final volume - Initial volume)
= 0.872 ( 1.39 - 4.40)
= 0.872 ( - 3.01)
= - 2.62 J
Therefore, the work done in the chemical reaction is - 2.62J
Learn more about work done, here:
https://brainly.com/question/16951089
#SPJ1
Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
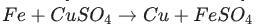
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
How much energy is produced in each stage cellular respiration and how is it used?

Answers
Answer:
Some of the energy from the electrons is used to pump hydrogen ions across the membrane, creating an electrochemical gradient that drives the synthesis of many more molecules of ATP. In all three stages of cellular respiration combined, as many as 38 molecules of ATP are produced from just one molecule of glucose.
Explanation:
True or False?
Sedimentary rocks can weather, erode and then become a different sedimentary rock, as seen in the rock cycle.
Answers
Answer:
True
Explanation:
The sedimentary particles from which a sedimentary rock is formed can be derived from a metamorphic, an igneous, or another sedimentary rock. Sedimentary rock can change into metamorphic rock or into igneous rock. Metamorphic rock can change into igneous or sedimentary rock.
Suppose the concentration of Al3+ is 1.90 M and the concentration of Sn2+ is 0.25 M in a galvanic cell that operates at 25°C with the same electrodes as the One above. Would the cell potential, Ecell, under these conditions be greater than, less than, or equal to the standard cell potential, E° cell from part b?
Justify your answer.
Answers
Under these conditions, the cell potential, Ecell, would be somewhat lower than the usual cell potential, E°cell.
How to determine cell potential?To determine whether the cell potential, Ecell, would be greater than, less than, or equal to the standard cell potential, E°cell, use the Nernst equation:
Ecell = E°cell - (RT/nF)ln(Q)
where:
Ecell = cell potential under the given conditions
E°cell = standard cell potential
R = gas constant (8.314 J/(mol·K))
T = temperature in Kelvin (25°C = 298 K)
n = number of electrons transferred in the overall cell reaction
F = Faraday constant (96,485 C/mol)
Q = reaction quotient
The balanced equation for the cell reaction is:
2Al(s) + 3Sn₂⁺(aq) → 2Al₃⁺(aq) + 3Sn(s)
The cell reaction involves the transfer of 3 electrons, so n = 3.
At standard conditions (1 M concentration for all species and 25°C), the cell potential, E°cell, can be calculated using the standard reduction potentials for the half-reactions:
Al₃⁺(aq) + 3e- → Al(s) E° = -1.66 V
Sn₂⁺(aq) + 2e- → Sn(s) E° = -0.14 V
E°cell = E°(cathode) - E°(anode) = (-0.14 V) - (-1.66 V) = 1.52 V
To calculate Q, use the given concentrations of Al3+ and Sn2+:
Q = ([Al₃⁺]²/[Sn⁺]³) = (1.90 M)² / (0.25 M)³ = 231.2
Now use the Nernst equation to calculate Ecell under the given conditions:
Ecell = E°cell - (RT/nF)ln(Q)
Ecell = 1.52 V - [(8.314 J/(mol·K))(298 K)/(3 mol)(96,485 C/mol)ln(231.2)]
Ecell = 1.52 V - (0.012 V) = 1.508 V
Therefore, the cell potential, Ecell, under these conditions would be slightly less than the standard cell potential, E°cell. This is because the concentration of Al₃⁺ is higher and the concentration of Sn₂⁺is lower than the standard conditions, which shifts the reaction towards the side with lower concentration of Al₃⁺ and higher concentration of Sn₂⁺. This means that the reaction is not occurring under standard conditions and the Nernst equation must be used to account for the non-standard conditions.
Find out more on concentration here: https://brainly.com/question/17206790
#SPJ1
(05.03 LC)
How many atoms of hydrogen are there in one molecule of water, if the chemical formula is H2O?
OO
오
01
02 03
Answers
suppose of ammonium sulfate is dissolved in of a aqueous solution of sodium chromate. calculate the final molarity of ammonium cation in the solution. you can assume the volume of the solution doesn't change when the ammonium sulfate is dissolved in it. round your answer to significant digits.
Answers
The final molarity of the ammonium cation in the solution is 0.066M.
Firstly, we need to calculate the number of moles of ammonium sulfate:
No of moles = mass(g)/molar mass
No of moles = 2.57/132.14
No of moles = 0.0195 mol
When ammonium sulfate dissolves in sodium chromate, the following reaction will take place:
(NH₄)₂SO₄ + Na₂CrO₄ ------> (NH₄)₂CrO₄ + Na₂SO₄
No of Moles of sodium chromate = molarity x volume
= 66 x 0.200
No of moles of Sodium Chromate = 0.0132 mol
According to the reaction, 1 mol of sodium chromate produces 1 mol of ammonium chromate.
In this reaction, the sodium chromate amount is less than ammonium sulfate.
It can be concluded that Sodium chromate is a limiting reagent.
Therefore 0.0134 mol of ammonium cation is present in the resulting solution.
Molarity of ammonium cation = 0.0134 mol / 0.2 L
Molarity = 0.066 M
Therefore, 0.066M is the Molarity of the ammonium cation.
To know more about the concentration of compounds, click here:
https://brainly.com/question/17206790
#SPJ4
What part of the scientific method involves controlling variables while testing a hypothesis? (2 points) Select one: a. Analyzing data b. Conducting an experiment c. Drawing a conclusion d. Making observations
Answers
Answer:
D
Explanation:
best choice, makes the most sense
In the scientific method, conducting an experiment involves designing and performing controlled experiments to test a hypothesis and the correct option is option B.
Scientists manipulate the independent variable while controlling other variables (dependent and controlled variables) to observe and measure the effect on the dependent variable.
By controlling variables, scientists can isolate the factors that influence the outcome, ensuring that any observed changes are a result of the manipulated variable and not other unrelated factors. This step allows for the collection of reliable data, which is essential for drawing meaningful conclusions based on the evidence gathered from the experiment.
Thus, the ideal selection is option B.
Learn more about Scientific method, here:
https://brainly.com/question/17309728
#SPJ3
Would a burning candle have potential or kinetic energy?
Answers
Answer:
When the candle is lit and burning, it has kinetic energy.
Explanation:
please smash brainliest on my doorstep
what is the functional group and homologous series of ethyl butyrate?
Answers
Answer:
Functional group: ROR'
Homologous series: ester [C6H12O2]
Mark brainliest please
Sometimes a simple incident in our lives can actually have a deeper meaning and impact on us than might be apparent at the time. Write a reflective narrative about a memorable experience in your life.
Answers
I remember the day when I was walking to the grocery store and saw an elderly woman sitting on the sidewalk with her cart. She looked tired and was trying to catch her breath. At first, I walked past her, not wanting to get involved. But then something made me stop and turn around. I approached her and asked if she needed any help. She told me that she had been walking for a while and needed to rest for a bit. I helped her sit down on a nearby bench, and we started talking.
As we talked, I learned that her name was Mary, and she had lived in the neighborhood for over 50 years. She shared stories of her life and her struggles, and I listened with empathy. I could see the gratitude in her eyes, and it made me realize the power of human connection.
That experience taught me that we should never underestimate the impact of a simple act of kindness. It made me more aware of the people around me and more willing to help others in need. It also showed me the importance of empathy and how it can make a difference in someone's life.
Looking back, that moment was a turning point for me. It made me more compassionate and more aware of the needs of others. It showedme that a small gesture of kindness can have a ripple effect and make a big difference in someone's life. It also reminded me that we all have our own stories and struggles, and sometimes all we need is someone to listen and show us a little empathy.
Since that day, I have made it a point to be more aware of the people around me and to offer help whenever I can. Whether it's holding the door open for someone or offering a listening ear, I try to be more present and empathetic in my interactions with others.
That experience also taught me the importance of slowing down and taking the time to connect with people. In our fast-paced world, it's easy to get caught up in our own lives and forget about those around us. But by taking the time to connect with others, we can create meaningful relationships and make a positive impact in the world.
Overall, that simple encounter with Mary taught me a valuable lesson about the power of empathy and human connection. It's a lesson that I will always carry with me and strive to live by in my daily life.
In English, you're asked to write a reflective narrative about a life event with a deeper impact than initially visible. Describe the event, your reaction, its deeper meaning, and how it shaped you.
Explanation:This question pertains to reflective writing, a style typically used in English subject coursework. The task is about developing a narrative based on a memorable experience in your life that had a deeper impact than initially visible. Begin by selecting an event, which while appearing simple, had profound implications in retrospect. Describe the event in detail (who, what, where, when), discussing your personal reaction at the time. Then, explain its deeper meaning or the impact it had on you. Drawing conclusions about how this event shaped your perspectives or behaviours would constitute the reflective element of your narrative.
Learn more about Reflective Narrative here:https://brainly.com/question/33018651
#SPJ2
5. In order for a bowling ball to move down the lane and strike pins, what must be applied to the ball?
(ICP.1.2)
A. A force less than any other forces that might act on the ball such as gravity or friction.
B. A force equal to any other force that might act on the ball such as gravity or friction.
C. A force greater than any other forces that might act on the ball such as gravity or friction.
D. A force that creates engugh friction for the ball to move down the lanes and hit the pins.
Answers
Answer:
c i think
Explanation:
You can change a subscript in an equation, but you cannot change the
coefficient.
O True
O False
Answers
Answer:
false
because you can't change a subscript ,
but you can change the coefficient.
which probing question lies within the scope of physics?
Answers
Physics is a vast field that addresses a wide range of questions about the nature of the physical world. Probing questions can help to explore this field and encourage critical thinking and deep exploration of its topics.
Probing questions are open-ended questions asked to gather information, encourage critical thinking and deep exploration of a particular topic. Physics is a natural science that studies matter and its motion through space-time. It is a branch of science that deals with the fundamental nature of the universe and seeks to explain how and why objects behave as they do in the physical world.The following are some examples of probing questions within the scope of physics:What is the nature of light-The nature of light is an important topic within the scope of physics. It refers to the dual nature of light, as both a wave and a particle. Light behaves as a wave when it is traveling through space and as a particle when it is interacting with matter.How do magnets work-Magnets are a common object in the world around us, and they have a broad range of applications. They work by producing a magnetic field, which can attract or repel other magnetic objects. This topic lies within the scope of physics.What is the relationship between energy and matter-Energy and matter are two fundamental concepts in physics. The relationship between them is described by Einstein's famous equation E=mc2, which states that matter and energy are two forms of the same thing and are interchangeable. The study of the relationship between energy and matter lies within the scope of physics.What is the nature of the universe?The study of the universe's nature is one of the most significant topics within the scope of physics. This question addresses the origins and properties of the universe, its components, and the laws that govern its behavior.
for such more questions on physical
https://brainly.com/question/1079154
#SPJ8
breaking glass is an example of what type of change
A. chemical
B. physical
C. element
D. compound
Answers
Answer:
B
Explanation:
B. Physical!
Hope this helps! Good luck, mate! :D
(a) Describe the process by which Nitrogen is obtained from air on a large scale
Answers
The element nitrogen exists as a gas and is obtained from air on a large scale by fractional distillation of air.
What is an element?An element is defined as a substance which cannot be broken down further into any other substance. Each element is made up of its own type of atom. Due to this reason all elements are different from one another.
Elements can be classified as metals and non-metals. Metals are shiny and conduct electricity and are all solids at room temperature except mercury. Non-metals do not conduct electricity and are mostly gases at room temperature except carbon and sulfur.
The number of protons in the nucleus is the defining property of an element and is related to the atomic number.All atoms with same atomic number are atoms of same element.
Learn more about element,here:
https://brainly.com/question/14347616
#SPJ2
If 25 mL of a HCl solution of unknown concentration was neutralized with 10 mL of a 0.30 M NaOH solution, what was the original concentration of the HCl solution
Answers
Answer:
0.12 M
Explanation:
Step 1: Write the balanced equation
NaOH + HCl ⇒ NaCl + H₂O
Step 2: Calculate the reacting moles of NaOH
10 mL of a 0.30 M NaOH solution react.
\(0.010L \times \frac{0.30mol}{L} = 3.0 \times 10^{-3} mol\)
Step 3: Calculate the reacting moles of HCl
The molar ratio of NaOH to HCl is 1:1. The reacting moles of HCl are 1/1 × 3.0 × 10⁻³ mol = 3.0 × 10⁻³ mol.
Step 4: Calculate the concentration of HCl
3.0 × 10⁻³ mol of HCl are in 25 mL of solution.
\(M = \frac{3.0 \times 10^{-3} mol}{0.025L} = 0.12 M\)