which of the given aldohexoses is dextrorotatory? d-glucose d-galactose d-gulose d-mannose cannot predict
Answers
The dextrorotatory aldohexose among the given options is d-glucose. The correct option is A.
Dextrorotatory means it rotates plane-polarized light to the right.
1. Identify the aldohexoses given: a. D-glucose, b. D-galactose, c. D-gulose, and d. D-mannose.
2. Consider the specific optical rotation values for each aldohexose. The positive value indicates dextrorotatory properties.
3. Compare the values and find the dextrorotatory aldohexose.
In this case, D-glucose has a positive specific optical rotation value, making it dextrorotatory.
To know more about aldohexose, refer here:
https://brainly.com/question/31180563#
#SPJ11
Related Questions
Explain why chemical reactions are used to form synthetic materials.
Answers
The rearrangement of the atoms is the reason why.
The synthetic materials form as a product due to chemical reactions with bonds breaking in the process; As the bonds break, the rearrange into these materials.
You're welcome :)
If the temperature of 1000 mL of gas is changed from -86oC to 101oC. What is the approximate final volume?
Answers
Answer:
2000 mL
Explanation:
We have the following data:
Initial volume: V₁ = 1000 mL
Initial temperature: T₁ = -86°C + 273 = 187 K
Final temperature: T₂= 101°C + 273 = 374 K
According to Charles' law, as the temperature of a gas is increased at constant pressure, the volume is increased. That is expressed mathematically as:
V₁/T₁ = V₂/T₂
Thus, we calculate the final volume V₂ as follows:
V₂ = V₁/T₁ x T₂ = (1000 mL)/187 K x 374 K = 2000 mL
Therefore, the final volume is 2000 mL.
Watered down koolaid is an example of
A. saturated solution
b. unsaturated solution
C. supersaturated solution
d. suspension
Answers
How many moles of sodium are required in order to produce 5 moles of
sodium chloride?
Answers
Answer:
5 moles of Sodium are required to produce 5 moles of Sodium Chloride.
Explanation:
I am assuming you were not given an equation and this is a synthesis reaction.
Let's begin by writing a balanced chemical equation for the synthesis of sodium chloride.
2Na⁺ + Cl₂⁻ -> 2NaCl
Since Cl₂ exists as a diatomic molecule, we must have 2 +1 charges from Na to balance out the 2 -1 charges of Cl₂ (-1 for each Cl atom). This will then form 2 moles of NaCl.
To produce 5 moles of sodium chloride, we can multiply the entire equation by 2.5. This will result in:
5Na⁺ + 2.5Cl₂⁻ -> 5NaCl
The stoichiometric ratios of this new equation is:
5 Na: 2.5 Cl₂: 5NaCl
5 moles of sodium (Na) are required to produce 5 moles of sodium chloride (NaCl).
Fructose does not undergo hydrolysis because it is a _____. a. Aldose. b. Hexose. c. Monosaccharide. d. Disaccharide. e. Polysaccharide.
Answers
Fructose does not undergo hydrolysis because it is a monosaccharide (option c).
Monosaccharides are the simplest form of carbohydrates, consisting of a single sugar unit. Fructose is a monosaccharide and is commonly known as a fruit sugar. It is a hexose (option b), meaning it has six carbon atoms.
Hydrolysis is a chemical reaction that involves breaking down a compound by adding water molecules. However, monosaccharides like fructose do not undergo hydrolysis because they cannot be further broken down into simpler sugars through the addition of water.
They are already in their simplest form and do not require hydrolysis for digestion or utilization in the body.
On the other hand, disaccharides (option d) and polysaccharides (option e) are more complex carbohydrates composed of multiple sugar units.
They can undergo hydrolysis, where the chemical bonds between the sugar units are broken by the addition of water, resulting in the formation of monosaccharides.
To know more about Monosaccharides refer here
brainly.com/question/1157431#
#SPJ11
Which point is the outlier on this scatter plot? What might the outlier represent?
A. (0.07, 18) might represent a newborn shark.
B. (0.07, 18) might represent an unusually large shark.
C. (105, 320) might represent an unusually large shark.
D. (105, 320) might represent a newborn shark.

Answers
Answer:
An outlier on a scatter plot represents something that doesn't fit.
The outlier on this scatter plot is D.(105, 320) might represent a newborn shark
Help please!! Use complete sentences to differentiate between acids and bases on the basis of their behavior when dissolved in water give an example of each type
Please put this in simple terms
Answers
Answer:
Bases and Acids are chemically opposite from each other,and there are multiple ways to distinguish how they react when dissolved in water.
Explanation:
Carl has an empty 35.0-liter tank that he wants to fill with 18.3 moles of oxygen gas. The gas in the tank will have a temperature of 25°C. If the air surrounding the tank is at standard pressure, what will be the gauge pressure of the oxygen in the tank in atmospheres?
Answers
The gauge pressure of the oxygen in the tank in atmospheres is 11.46 atm.
What is gauge pressure?
The gauge pressure is given as the pressure relative to the ambient atmospheric pressure. The gauge pressure can be calculated as:
Gauge pressure = System pressure - Atmospheric pressure
The system pressure of the 35-liter tank with 18.3 moles oxygen is given as:
Pressure = moles * Rydberg constant * Temperature / Volume
Pressure = 18.3 moles * 0.08 L.atm.mol⁻¹.K⁻¹ * 298 K / 35 L
Pressure = 12.46 atm
The standard pressure is given as 1 atm.
Thus, the gauge pressure of the oxygen in the tank can be:
Gauge pressure = System pressure - Atmospheric pressure
Gauge pressure = 12.46 atm - 1 atm
Gauge pressure = 11.46 atm
The gauge pressure of the oxygen in the tank in atmospheres is 11.46 atm.
Learn more about gauge pressure, here:
https://brainly.com/question/15734722
#SPJ1
Answer:
The answer is 8.31 atm.
Explanation:
Ideal Gas Law = PV = nRT
(where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.)
First, convert the temperature from Celsius to Kelvin:
25°C + 273.15 = 298.15 K
Next, plug in the known values into the Ideal Gas Law equation:
P * 35.0 L = 18.3 moles * 8.31 J/mol*K * 298.15 K
Finally, solve for P:
P = (18.3 moles * 8.31 J/mol*K * 298.15 K) / 35.0 L
The pressure is in units of joules per liter. To convert it to atmospheres, divide by the atmospheric pressure in joules per liter:
1 atm = 101,325 Pa = 101,325 J/m^3 = 101.325 kPa
P (in atm) = (18.3 moles * 8.31 J/mol*K * 298.15 K) / (35.0 L * 101.325 kPa)
= 8.31 atm
Please balance these chemical equations
Answers
The chemical equations given would be balanced below
How should a chemical equation be balanced?The balanced equation of the various given chemical equations can be determined only when the number of atoms on the product part is the same with the number of atoms on the reactant side.
For 1.)
\(N_{2} + 3H_{2} ----- > 2NH_{3}\)
For 2.)
\(2KCl_{3} ----- > 2KCl + 3O_{2}\)
For 3.)
\(2NaCl + F_{2} ----- > 2NaF + Cl_{2}\)
For 4.)
\(2H_{2} + O_{2} ----- > 2H_{2} O\)
For 5.)
\(Pb(OH)_{2} + 2HCl ----- > 2H_{2} O +PbCl_{2}\)
For 6.)
\(2AlBr_{3} + 3K_{2}SO{4} ---- > 6KBr + Al_{2}(SO_{4})_{3}\)
For 7.)
\(CH_{4} + O_{2} ----- > CO_{2} + 2H_{2} O\)
For 8.)
\(C_{3} H_{8} + 3O_{2} ----- > 3CO_{2} + 4H_{2} O\)
For 9.)
\(C_{8} H_{18} + 8O_{2} ----- > 8CO_{2} + 9H_{2} O\)
Learn more about chemical equation here:
https://brainly.com/question/30265799
#SPJ1
experiments also show that any aqueous solution at 25 degree celsius, the ionic-product of water Kw is equal to a constant value:
Answers
In any aqueous solution at 25°C, the ionic-product of water (Kw) is a constant value equal to 1.0 x 10⁻¹⁴ mol²/L².
Due to the auto-ionization or self-ionization of water, water molecules dissociate into hydronium ions (H₃O⁺) and hydroxide ions (OH⁻). At 25°C, the Kw is equal to 1.0 x 10⁻¹⁴ mol²/L².
This constant value of Kw plays a crucial role in understanding the acidity and basicity of aqueous solutions. It helps to establish the relationship between the concentrations of hydronium and hydroxide ions, as their product remains constant at a given temperature. The pH and pOH scales are derived from this relationship, providing a convenient method for measuring the acidity or basicity of a solution.
In summary, the ionic-product of water, Kw, remains constant at 1.0 x 10⁻¹⁴ mol²/L² for any aqueous solution at 25°C. This constant is a result of the auto-ionization of water and helps to understand the relationship between hydronium and hydroxide ions in the context of acidity and basicity.
Learn more about ionic-product of water here: https://brainly.com/question/31640554
#SPJ11
if 2.86 moles of sodium hydroxide were added to water to create a solution that is 0.858 M, what is the volume of the solution?
Answers
Molarity = moles of solute / volume of solution in liters
We are given the moles of solute (2.86 moles) and the molarity (0.858 M). Let's rearrange the formula to solve for the volume:
Volume of solution = moles of solute / molarity
Volume of solution = 2.86 moles / 0.858 M
Volume of solution = 3.33 L
Therefore, the volume of the solution is 3.33 liters.
ILL GIVE BRAINLIEST
There are 164 g H3PO3 formed during a
reaction. How many moles of H₂O are
required? (H3PO3: 82 g/mol)
P₂O3 + 3H₂O → 2H3PO3
164 g H3PO3|
164 g H3PO3 → [?] mol H₂O

Answers
164 g of H₃PO₃ (phosphorus acid) would require 3 moles of H₂O for the reaction.
Given information,
Mass of H₃PO₃ = 164g
Moles of H₃PO₃ = 82 g/mol
The balanced chemical equation:
P₂O₃ + 3H₂O → 2H₃PO₃
For every 2 moles of H₃PO₃ produced, 3 moles of H₂O are required.
The number of moles of H₃PO₃:
Moles of H₃PO₃ = mass of H₃PO₃ / molar mass of H₃PO₃
Moles of H₃PO₃ = 164 g / 82 g/mol
Moles of H₃PO₃ = 2 mol
The number of moles of H₂O:
Moles of H₂O = (3/2) × moles of H₃PO₃
Moles of H₂O = (3/2) × 2 mol
Moles of H₂O = 3 mol
Therefore, 164 g of H₃PO₃ would require 3 moles of H₂O for the reaction.
Learn more about moles, here:
https://brainly.com/question/30885025
#SPJ1
What masses of ethylamine (Kb = 5.60 × 10–4 ) and ethylammonium chloride do you need to prepare 1.31 L of pH = 10.792 buffer if the total concentration of the two components is 2.34 M?
Ethylamine:
Ethylammonium chloride:
Answers
We need 3.50 g of ethylamine and 240.29 g of ethylammonium chloride to prepare 1.31 L of pH = 10.792 buffer with a total concentration of 2.34 M.
Ethylamine: 3.50 g Ethylammonium chloride: 240.29 g
Implement Henderson-Hasselbalch equationTo prepare 1.31 L of pH = 10.792 buffer with a total concentration of 2.34 M, we need to use the Henderson-Hasselbalch equation:
pH = pKa + log([base]/[acid])
First, we need to find the pKa of ethylamine using its Kb value:
pKa = -log(Ka)
= -log(Kw/Kb)
= -log(1.0 × 10⁻¹⁴/5.60 × 10⁻⁴)
= 9.208
Now we can plug in the values into the
Henderson-Hasselbalch equation:
10.792 = 9.208 + log([base]/[acid]) 1.584
= log([base]/[acid]) 10¹·⁵⁸⁴
= [base]/[acid] 38.75 = [base]/[acid]
Since the total concentration of the two components is 2.34 M, we can set up a system of equations:
[base] + [acid] = 2.34 [base]/[acid] = 38.75
Solving for [base] and [acid], we get:
[base] = 2.34/(38.75 + 1) = 0.0592 M [acid] = 2.34 - 0.0592 = 2.2808 M
Now we can find the masses of ethylamine and ethylammonium chloride needed:
Mass of ethylamine = (0.0592 M)(1.31 L)(45.08 g/mol) = 3.50 g
Mass of ethylammonium chloride = (2.2808 M)(1.31 L)(80.54 g/mol) = 240.29 g
Learn more about ethylamine at
https://brainly.com/question/9439418
#SPJ11
T/F: The electromagnetic force holds negatively charged electrons in orbit around thr positively charged nucleus of an atom
Answers
Answer:
Explanation:
Oppositely charged particles attract each other, while like particles repel one another. Electrons are kept in the orbit around the nucleus by the electromagnetic force, because the nucleus in the center of the atom is positively charged and attracts the negatively charged electrons.
Balance the following equation:
_Mg + HNO3 → Mg(NO3)2+ _H₂
Answers
The balanced equation of the reaction is:
Mg + 2HNO₃ → Mg(NO₃)₂ + H₂What is a balanced equation of reaction?To balance the chemical equation:
Mg + HNO₃ → Mg(NO₃)₂ + H₂
We need to ensure that the number of atoms of each element is the same on both sides of the equation.
The balanced equation is:
Mg + 2HNO₃ → Mg(NO₃)₂ + H₂
By adding a coefficient of 2 in front of HNO₃ and a coefficient of 2 in front of H₂, we balance the equation.
This ensures that there are two nitrogen atoms, six oxygen atoms, four hydrogen atoms, and one magnesium atom on both sides of the equation.
Learn more about balanced equations at: https://brainly.com/question/11904811
#SPJ1
questionyou heat two substances, a and b. both substances change color. when cooled, both substances return to their original colors.what most likely happened in this situation?
Answers
Two substances, a and b were heated. Both substances change color. when cooled, both substances return to their original colors.
What is a physical change?
A physical alteration only affects the object's shape; the object's interior structure (its molecules) remain unaltered. It is possible to change the created element back into the original thing. A alteration that can be undone is reversible. The condition, shape, or size of an object can change due to a physical change.
Here, since after heating both the substances a and b change colour and again on cooling return back to their original form (previous colour), thus we can conclude that a physical change occurs in them on heating which reverts back on cooling, being a reversible process.
To learn more about physical change from the given link below,
https://brainly.com/question/17609067
#SPJ4
why are the oxidation numbers of alkali metals always positive?
Answers
The oxidation numbers of alkali metals are always positive because they have only one electron in their outermost shell. This means that they are highly reactive and readily lose this electron to form a positive ion.
What are the alkali metals?The alkali metals include lithium, sodium, potassium, rubidium, cesium, and francium.
All of these elements have an oxidation number of +1 because they all have one valence electron that they are likely to lose in a chemical reaction. This is why the oxidation numbers of alkali metals are always positive.
Learn more about alkali metals here https://brainly.com/question/19109836
#SPJ11
how can coffee be turned back to water
Answers
Answer:
distillation, collecting and cooling the steam
Explanation:
How many senses does the human body have?
O A. 3
B. 5
Answers
Answer:
B
Explanation:
sight, hearing, smell, taste and touch
Answer:
Most easiest answer..
There are 5 senses in human body.☺️
how to determine the molar mass of an unknown acid
Answers
To determine the molar mass of an unknown acid, follow these steps:
1. Obtain the formula of the unknown acid.
2. Calculate the mass of a sample of the acid.
3. Determine the number of moles of the sample of the acid.
4. Determine the number of grams per mole of the acid.
The molar mass of the unknown acid can be calculated by dividing the mass of the sample of the acid by the number of moles of the sample of the acid. Molar mass is defined as the mass of one mole of a chemical substance. It is usually expressed in grams per mole. It is calculated by adding up the atomic weights of each of the atoms in the chemical formula of the substance. For instance, let's suppose you want to calculate the molar mass of an unknown acid, HA. The first step is to obtain the chemical formula of the acid, which is HA.
Then, using a balance, obtain the mass of a sample of the acid, for instance, 0.5 g. To determine the number of moles of the sample of the acid, you'll need to divide the mass of the sample by the molar mass of the acid.To determine the number of grams per mole of the acid, add up the atomic weights of each of the atoms in the chemical formula of the substance.
To know more about molar mass refer to:
https://brainly.com/question/837939
#SPJ11
PLEASE HELP! I will give brainliest!
4. You have knowledge of the outermost electron states of the atoms, trends in the periodic table, and the patterns of chemical properties. Use that knowledge to predict the products of each of the following equations. Then explain how you made your predictions. Be sure to balance the equations. (4 points)
a. ___NaBr + ___Ca → _______ + _______
b. ___Mg + ___O2 → _______
Answers
The reactions are completed as folows;
2NaBr + Ca -----> CaBr2 + Na
2Mg + O2 ----> 2MgO
What is the electrochemical series?The electrochemical series has to do with the arrangement of atoms in order of reactivity. The metals that are up in the series are more reactive than the metals that are below.
In this case, the reactions are completed as folows;
2NaBr + Ca -----> CaBr2 + Na
2Mg + O2 ----> 2MgO
Learn more about chemical reaction:https://brainly.com/question/14953274
#SPJ1
Energy that carries sound
Answers
.........
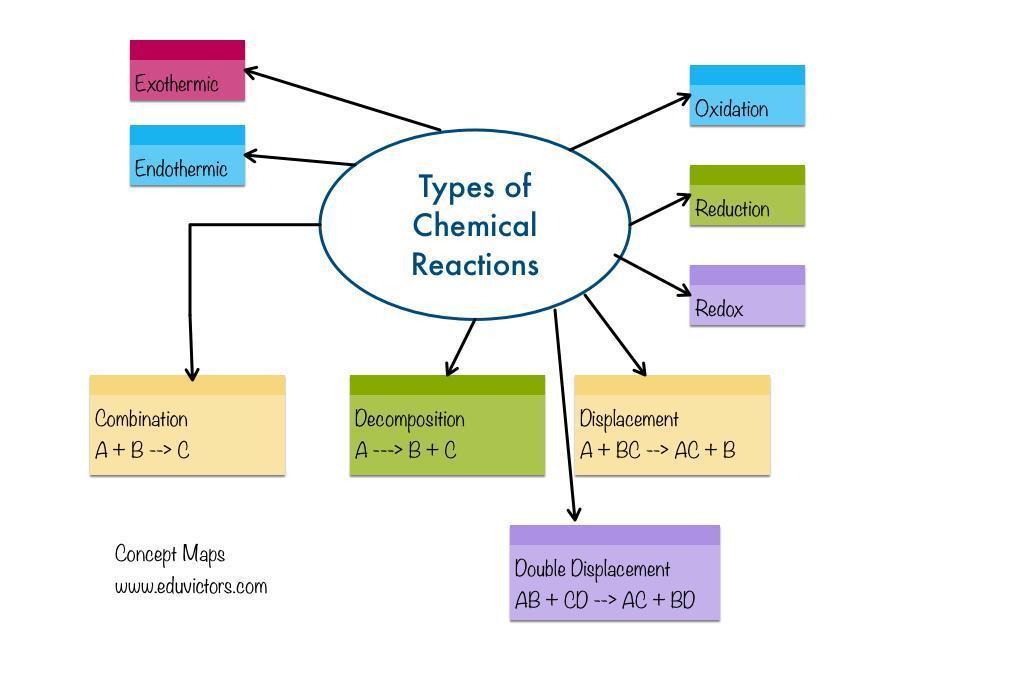
chemical change grade 10 mind map
Answers
Chemical change: A chemical reaction is the change of one chemical substance into another chemical substance. For instance: The rusting of iron, the curdling of milk, the digestion of food, breathing, etc.
What is a chemical reaction?
A chemical reaction results in a chemical change because a new material has entirely different properties from the original substance. In a chemical reaction, atoms rearrange themselves.Reactants are the chemicals that participate in a chemical reaction.Products are the new compounds created as a result of a chemical process. An illustration of a chemical reaction is burning magnesium in the air to produce magnesium oxide.2Mg(s) + O2(g) △→ 2MgO(s)The magnesium ribbon is cleaned with sandpaper before being burned in the air. This cleans the magnesium ribbon's surface of the basic magnesium carbonate protecting coating.Reactant: Materials that participate in a chemical reaction are referred to as reactants. Mg and O2, as an example.A product is a newly created substance that results from a chemical reaction. Example: MgO.A chemical reaction is the change of one chemical substance into another chemical substance.
To learn more about chemical reactions, refer to:
https://brainly.com/question/1222323
#SPJ9

Chemical change is the change chemical substance is transformed into another chemical substance.It is irreversible in nature , for example Reaction of medicine in body , milk to curd etc.
What is the difference between chemical and Physical change?1)Physical change temporary or reversible in nature but chemical change is irreversible in nature
2)In physical change there no new product is formed but in chemical change there formation of new product takes .
3) Physical change is change sin shape ,size or state for example freezing of water , melting of wax , and example of Chemical change are Burning of coal, digestion of food
to learn more about chemical change click here https://brainly.com/question/28089135
#SPJ9
Problem: Co3+ | Co2+ and Ni2+ | NiAnode?Cathode?(You need to use Reference Table B-16.)a. Co2+b. can't answerc. Ni2+d. Nie. Co3+

Answers
Answer:
- Anode: Co3+ | Co2+
- Cathode: Ni | Ni2+
Explanation:
The anode is where oxidation reaction occurs, and the cathode is where reduction reaction occurs.
From the table of reduction potencials, we find that:
- Co reaction:
\(\begin{gathered} Co^{3+}+2e^-\rightarrow Co^{2+} \\ E=1.81\text{ }V \end{gathered}\)- Ni reaction:
\(\begin{gathered} Ni\rightarrow Ni^{2+}+2e^- \\ E=-0.250\text{ V} \end{gathered}\)Now, to find out which one is the anode and which one is the cathode, it is necessary to compare the reduction potencials.
The reaction of Ni have negative potentials, so Ni will be the anode and Co will be the cathode.
One molecule of silver atoms contains the same number of items found in one mole of calcium atoms. TrueFalse
Answers
When they talk about items found, they refer to molecules. According to Avogadro, one mole of any substance, regardless of its type, contains the same amount of molecules equal to 6.022x10^23.
Now they tell us about a molecule of silver and a mole of calcium. In one mole of calcium, there will be 6.023x10^23 molecules, which is different from one molecule of silver, so the answer will be false
What is the mass number of an atom that has 12 neutrons and 11 protons?
24
12
11
23
Answers
Answer:
23
Explanation:
Which of the following statements is FALSE?
The microscopic size of cells enable them to function properly.
Most organisms on Earth are multi-celled.
Some cells can be shaped like a ball.
Growing children’s cells are constantly dividing.
Answers
Answer:
The answer is functioning properly ok dude?
Explanation:
Answer: "The microscopic size of cells enable them to function properly.
Most organisms on Earth are multi-celled." is false
Explanation:
In unicellular organisms, cell division is the means of reproduction; in multicellular organisms, it is the means of tissue growth and maintenance. Survival of the eukaryotes depends upon interactions between many cell types, and it is essential that a balanced distribution of types be maintained. This is achieved by the highly regulated process of cell proliferation. The growth and division of different cell populations are regulated in different ways, but the basic mechanisms are similar throughout multicellular organisms.
Determine the molarity of a solution formed by dissolving 0.968 g of MgCl2in enough water to yield 150.0 mL of solution.
Answers
Answer:
M = 0.0678 M
Explanation:
Hello there!
In this case, since the molarity of a solution is obtained by dividing the moles by the volume, it is firstly necessary to compute the moles of magnesium chloride in 0.968 g, via its molar mass, as shown below:
\(n=\frac{0.968g}{95.21g/mol}=0.0102mol\)
Next, since 150.0 mL in liters is 0.1500 L, according to the appropriate units, the resulting molarity is:
\(M=\frac{0.0102mol}{0.1500L}\\\\M=0.0678M\)
Best regards!
The types of atoms found in a glucose molecule include all of the following except
a. carbon.
b. oxygen.
c. nitrogen.
d. hydrogen
Answers
Glucose is a fundamental carbohydrate and a monosaccharide with the molecular formula C6H12O6. The types of atoms found in a glucose molecule include all of the following except nitrogen, so the correct answer is c. nitrogen.
Atoms are the smallest unit of an element that has the chemical characteristics of that element. The glucose molecule is made up of carbon, hydrogen, and oxygen atoms. It contains six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. Glucose is a source of energy that is used by organisms. It's produced by photosynthesis in plants and is used as a source of energy in animal cells.Glucose is a monosaccharide, which means that it is a simple sugar. Other monosaccharides include fructose and galactose, which are isomers of glucose. It's a key component in many carbohydrate-containing foods such as bread, pasta, and potatoes. In the human body, glucose is used to provide energy to cells, and excess glucose is stored in the liver and muscles as glycogen. Glucose is also used as a sweetener in many foods and drinks.
To know more about Glucose visit :
brainly.com/question/13555266
#SPJ11
help plz will mark brainliest xD

Answers
Answer:
Ions are atoms that have either gain or lose electrons from the neutral atom.
A loss of electrons results in a positive ion, called a cation, a gain of electron results in a negative ion called an anion.
Ionic compounds are formed by the combination of a cation and anion, or metal + nonmetal.
When naming ionic compounds, name the cation first, and the anion second, changing the ending to ide.
Explanation:
Hope it helped!
Answer:
• [ lost ] or [ gained ]
• [ cation ], [ anion ]
• [ metal ] + [ non-metal ]
• [ cation ] and the [ anion ] to [ compound with suffix "ide" or "ode" ]