Which of the following molecules contains an ionic bond? MgCl2. Cl2 SF3 Cl2O7 none of the above.
Answers
MgCl₂ contains an ionic bond. Ionic bonds are formed between atoms with significant differences in electronegativity.
In an ionic bond, one atom donates electrons to another atom, resulting in the formation of ions.
Among the options provided, MgCl₂ is the molecule that contains an ionic bond. Magnesium (Mg) is a metal with a low electronegativity, while chlorine (Cl) is a nonmetal with a relatively high electronegativity. In MgCl₂, magnesium donates two electrons to each chlorine atom, forming two chloride ions (Cl⁻) and one magnesium ion (Mg²⁺). The electrostatic attraction between the positively charged magnesium ion and the negatively charged chloride ions creates the ionic bond.
In contrast, Cl₂ (chlorine gas) consists of two chlorine atoms sharing electrons in a covalent bond. SF₃ (sulfur trifluoride) also involves covalent bonds, where sulfur and fluorine atoms share electrons. Cl₂O₇ (dichlorine heptoxide) is a covalent compound as well, with chlorine and oxygen atoms sharing electrons.
learn more about ionic bond Here:
https://brainly.com/question/12663276
#SPJ4
The molecule that contains an ionic bond is MgCl2.
An ionic bond is formed when there is a transfer of electrons between atoms, resulting in the formation of ions. In the given options, MgCl2 is the molecule that contains an ionic bond.
MgCl2 consists of magnesium (Mg) and chlorine (Cl) atoms. Magnesium donates two electrons to chlorine, resulting in the formation of Mg2+ cation and two Cl- anions. The electrostatic attraction between the oppositely charged ions forms the ionic bond in MgCl2.
On the other hand, Cl2, SF3, and Cl2O7 do not contain ionic bonds. Cl2 is a diatomic molecule composed of two chlorine atoms sharing a covalent bond. SF3 is a molecule with covalent bonds between sulfur and fluorine atoms. Cl2O7 is a covalent compound formed by the sharing of electrons between chlorine and oxygen atoms.
Learn more:About molecules here:
https://brainly.com/question/19922822
#SPJ11
Related Questions
Which type of force caused the weight to drop (11) after the rope was cut?
Friction Force
Normal Force
Applied Force
Electrical Force
Gravitational Force
Magnetic Force

Answers
Answer:
gravitational force.
How many moles is 6.88 x 10^-2 g silver chloride ?
Answers
Answer:hey
Explanation:
lmk, due in 30min!!!!!!!!!!!!!!!!

Answers
The conclusion that is consistent with the explanation is that substance C is a mixture because it contains 2 different compounds. Option C.
What are mixtures?Mixtures are substances that contain two or more different substances mixed together. These mixed substances do not react together but maintain their chemical identities such that each can be recovered by physical means.
Thus, when a mixture is examined, it can be found to contain more than one compound. These mixtures are separatable by physical separation methods such as filtration, evaporation, decantation, etc.
Thus, the conclusion that goes with the right explanation is that substance C is a mixture because it contains 2 different compounds.
Option A is incorrect because mixtures do not require running an electric current through them for separation. B is incorrect because a substance being consistent does mean that it is pure. D is incorrect because a substance being pure does not mean it won't react.
More on mixtures can be found here: https://brainly.com/question/24898889
#SPJ1
Perform the following operation
and express the answer in
scientific notation.
(7.296 × 10²) = (3.6 × 10-⁹)
[?]
? ] × 10°
Answers
After perform the following operation and express the answer in scientific notation is 7.6×10¹⁰
What is scientific notation?
Scientific notation is a way to present extremely large or extremely small numbers in a more understandable way. We are aware that full numbers can go on forever, but we are unable to write such enormous figures on paper. Additionally, a simpler method of representation was required for the numbers that appear at the millions place after the decimal. This makes it challenging to represent a small number of integers in their enlarged form. We therefore employ scientific notations. Learn general form for numbers as well. In scientific notation, 100000000 can be written as 108, for instance. Exponent is positive in this case. Similar to 0.0000001, which has a negative exponent and is a very small number, 10-8 can be used to symbolise it.
=7.296×10²÷9.6×10⁻⁹
=7.296×10²/9.6×10⁻⁹
=0.76×10¹¹
=7.6×10¹⁰
To learn more about scientific notation
https://brainly.com/question/1767229
#SPJ9
Uranium-238 had a half life of 4.5 billion years. A 100 g sample of U-238 has decayed until only 25 g remain. How long did it take for the sample to decay? (Write the answer in “n billion years”)
Answers
awnseris chemical hhhh Explanation:
Mass is a(n)
property.
O a. electrical
Oc. chemical
Ob. physical
O d. natural
Answers
Answer:
d. natural I think is the right answer
Explanation:
I think thats the right answer
A student was given a mixture of iron of iron fillings and sulphur. He was told to heat it and observe the compound.
(a) What is coloured formed.
(b) Write the effect of magnet on it.
(c) Write the action of carbon disulphide on it.
(d) Describe the effect of adding dilute hydrochloric acid to it. ldentify the gas and write its two properties.
Answers
The observations are as follows:
black iron sulfide is formed iron sulfide is not magnetic No reaction observed with carbon sulfidehydrogen gas is evolved What is the product of the reaction of iron fillings and sulfur when heated?When a mixture of iron fillings and sulfur are heated, black iron (ii) sulfide is formed.
Unlike iron fillings, black iron (ii) sulfide is not attracted to a magnet.
When carbon sulfide is added to it, no visible reaction is observed.
When dilute hydrochloric acid is added to it, a gas with a rotten-egg smell is evolved.
The gas is hydrogen sulfide which is a reducing agents and acidic in nature.
Learn more about hydrogen sulfide at: https://brainly.com/question/15842521
#SPJ1
Rank the following diatomic species of carbon in order of bond length and bond strength. A. C B. C2 C. C 1)Bond Length -> Longest Next Shortest 2)Bond Length -> Longest Next Shortest
Answers
1) Bond Length -> Longest Next Shortest:
A. C > C2 > C
2) Bond Strength -> Strongest Next Weakest:
A. C2 > C > C
In general, bond length is inversely proportional to bond strength. A shorter bond means a stronger bond, while a longer bond means a weaker bond. When comparing the diatomic species of carbon, we can assume that the bond strength and bond length are related in the same way.
A) C: The bond length in a C-C bond is about 1.54 angstroms (Å), which is considered a relatively strong bond. Since this is a single bond, we can assume that it is longer than the bonds in the other two diatomic species.
B) C2: The bond length in a C=C bond is about 1.34 Å, which is shorter than the bond length in C-C. Since C2 has a triple bond, it should have a shorter bond length and a stronger bond than C.
C) C: The bond length in a C≡C bond is about 1.20 Å, which is the shortest of the three species. The triple bond in C has the shortest bond length and the strongest bond strength.
Therefore, the order of bond length from longest to shortest is A, B, C, and the order of bond strength from weakest to strongest is C, B, A. The order of bond strength and bond length are related in the same way because the strength of the bond is proportional to the bond length.
Know more about bond length here:
https://brainly.com/question/24404422
#SPJ11
Help needed fast, please ?

Answers
The standard reduction potential for the half-reaction of Be^2+ + 2e^- -> Be is given as E^0 = 3.83 V.
For the half-cell Hg^2+ | Hg, the standard reduction potential is not provided in the given information. To calculate the electric potential for the voltaic cell, we need the reduction potential for the Hg^2+ | Hg half-cell.
A voltaic cell, also known as a galvanic cell or an electrochemical cell, is an electrochemical device that generates electrical energy through a spontaneous chemical reaction. It consists of two half-cells connected by an external circuit and a salt bridge or porous barrier that allows the flow of ions between the two half-cells.
Each half-cell consists of an electrode immersed in an electrolyte solution. The electrode is typically made of a metal or a conductive material, and the electrolyte is a solution containing ions that can participate in the redox (reduction-oxidation) reaction.
Learn more about electrochemical cell on:
https://brainly.com/question/23631454
#SPJ1
which planet has a hot, turbulent atmosphere dominated by carbon dioxide?
Answers
Answer:
Neptune
Hope this helps :)
Pls brainliest...
Why are all molecules not compounds?

Answers
Answer:
Explanation:
All compounds are molecules, but not all molecules are compounds. That is because a molecule can be made up of two atoms of the same kind, as when two oxygen atoms bind together to make an oxygen molecule. However, all compounds are made up of two or more different types of atoms.
Answer:
the last one
Explanation:
Electrical charge of an alpha particle is,
A. +1
B. +2
C. +3
D. +4
Answers
How many moles of sulfuric acid are required to neutralize 0.70 mol of sodium hydroxide?
Answers
answer and explanation
to correctly answer this question we first need to look at the mol ratio of the balanced stoichiometric equation
H₂SO₄ + 2NaOH → Na₂SO₄ + 2H₂O
from this balanced equation we see that 2 mols of NaOH will be neutralized by 1 mol of sulfuric acid. therefore the mols ratio is
H₂SO₄: NaOH
1:2
if we have 0.70 mol of NaOH then we need :
(0.70 x1)/2 = 0.35 mols of sulfuric acid
I only need the answer to numberr 4

Answers
The substance that is produced from a chemical reaction
•reactants
•chemical reaction
•atom
•product
•molecule
Answers
Answer - The substance that is produced from a chemical reaction....
REACTANTS
How many electrons does chlorine need to obtain a full outer energy level of electrons? A) 1 B) 2 C) 3 D) 4

Answers
You just need one to reach full energy. (A)
Only one electrons does chlorine need to obtain a full outer energy level of electrons. Thus, option A is correct.
What is an electron?An electron is defined as a subatomic particle which is charged negatively either bounded to an atom or not it means free. The nucleus of an atom is made up of electron, proton and neutron. The electron is a basic particle and electron is not made of anything.
The nature of electron is free it means they are present freely in nature and consist of an atom.The main function of electron is that they play the role of negatively- charged component of an atom.
The electrons are primary source of current conducting and electrons number as well as atomic number of an element is always same or equal. There is no size of electron but the mass of electron is 9×10^-31.
Learn more about electron here:
https://brainly.com/question/1255220
#SPJ6
um.. i dont need help lol
Answers
Answer:
thats cool mate
Explanation:
hope ya have a good day, im answering just for the points tbh
Answer:
ello thats great free points then >:3
-XxanimexX
Which of the following is correct about silicon?
A.It is a metalloid
B.The blue grey colour with metallic lustre
C.It is used as a semiconductor
D.All of these
Answers
The correct statement about silicon is D. All of these.
Silicon (Si) is a chemical element that exhibits properties of a metalloid. A metalloid is an element that has characteristics of both metals and nonmetals. Silicon has a shiny, blue-grey color with a metallic luster, which is characteristic of many metalloid elements.
Additionally, silicon is widely used as a semiconductor material. Semiconductors have electrical conductivity between that of a conductor and an insulator. Silicon's ability to conduct electricity can be modified by adding impurities, a process known as doping. This property makes silicon a fundamental component in the manufacturing of electronic devices such as transistors, integrated circuits, and solar cells.
Therefore, all of the given options are correct. Silicon is a metalloid, it has a blue-grey color with metallic luster, and it is commonly used as a semiconductor in various technological applications.
learn more about silicon here:
https://brainly.com/question/30367432
#SPJ11
Compare and contrast the political system
(institutions, branches of government, electoral rules) of France
and Russia. How do they compare? What are the key distinguishing
features? What are the stre
Answers
Russia is a federation with a semi-presidential political system. The President is the head of state while the Prime Minister is the head of government. The Federal Assembly is a bicameral legislature that is made up of the State Duma (lower house) and the Federation Council (upper house).
The political system in Russia and the United States are different. In the US, it is a presidential system where the President is both the head of state and government, while in Russia, the President is the head of state while the Prime Minister is the head of government.
In the US, the Congress is made up of the Senate (upper house) and the House of Representatives (lower house) while in Russia, the Federal Assembly is made up of the State Duma (lower house) and the Federation Council (upper house).
The key distinguishing features between the political systems in Russia and the US include the role of the President, the structure of the legislature, and the nature of the judiciary. In Russia, the President has a lot of power and is able to appoint the Prime Minister and other members of the executive branch.
The judiciary is also less independent compared to that of the US. On the other hand, the US has a more balanced system of power between the three branches of government, with the judiciary being independent of the executive and legislative branches.
The strengths of the political system in Russia include a strong centralized government that is able to make quick decisions and a strong military. However, the lack of political pluralism and the weak judiciary system are key weaknesses of the system.
The US political system has a strong commitment to individual rights and democratic principles. However, the system is often characterized by gridlock and polarization between political parties, leading to slow decision-making and a lack of progress on important issues.
To know more about Federal Assembly here
https://brainly.com/question/28863069
#SPJ11
Jim uses an electric balance to measure the mass of a magnesium strip. If the strip has a mass of 6.10 g, how many moles of magnesium are in the strip?
Answers
The number of moles of magnesium that are in the strip is 0.25 mole
From the question:
We are to determine number of moles of magnesium that are present in the strip
Using the formula
\(Number\ of\ moles = \frac{Mass}{Atomic\ mass}\)
Mass of magnesium strip = 6.10 g
Atomic mass of magnesium = 24.305 g/mol
∴ Number moles of magnesium present = \(\frac{6.10}{24.305}\)
Number moles of magnesium present = 0.250977 mole
Number moles of magnesium present ≅ 0.25 mole
Hence, the number of moles of magnesium that are in the strip is 0.25 mole.
Learn more on calculating number of moles here: https://brainly.com/question/14464650
Your team is assigned the Funky Mix. Your unknown has a boiling range of 121-124 oC. You take an IR of your compound and see a carbonyl peak at 1730 cm -1. What is the most likely identity of your unknown
Answers
It is highly probable that your unknown compound is a ketone.
Based on the information provided, the most likely identity of your unknown compound is a ketone.
The presence of a carbonyl peak at 1730 cm-1 in the IR spectrum suggests that the compound contains a carbonyl functional group, which is commonly found in ketones.
Additionally, the boiling range of 121-124 oC is consistent with the boiling range of many ketones. Therefore, it is highly probable that your unknown compound is a ketone.
to learn more about ketone click here:
brainly.com/question/31044163
#SPJ11
A cube of zinc and a cube of silver have the same volume. The mass of the zinc is 55lbs (pounds). What is the mass of the silver in kg (kilograms)?
Answers
Answer:
Explanation:
Convert 55lbs to kg
(55 lb)*(453.6 g/lb) = 24948 g or 249.48 kg
Density of zinc = 7140 kg/m^3
Find the volume occupied by 249.48 kg:
(249.48 kg)/(7140 kg/m^3) = 0.055 m^3
Density of silver = 10,490 kg/m^3
Mass of Ag in 0.055m^3:
(0.055m^3)*(10,490 kg/m^3) = 577 kg
Limonene is an oil from oranges and lemons. Click on all the carbon
atoms its structures that have a trigonal planar molecular geometry.
Answers
Answer:
See explanation
Explanation:
Limonene is an oil from oranges and lemons. Its structure has been shown in the image attached to this answer.
There are two different groups of carbon atoms in limonene. One group of carbon atoms are sp2 hybridized while the other group are sp3 hybridized. The group of carbon atoms that are sp2 hybridized have a trigonal planar geometry and have been accurately labeled in the image attached while the carbon atoms that are sp3 hybridized were also labelled and have a tetrahedral geometry.
Hence in limonene, there are trigonal planar and tetrahedral carbon atoms.

Which of these would most likely happen to a species that is not able to adapt to a changing environment?
Answers
Answer:
If a species cannot adapt to the changes in their ecosystem, they may move to another location. If they will not move, the species may become threatened, endangered or extinct.
Explanation:
The way the question is worded, it sounds like there are options for you to chose from that you are not showing. I could help you out more if I actually knew the options.
Extinction is likely if conditions change faster than a species can evolve, and if members of that species lack the traits required to survive in the new environment.
What is an extinction ?The death of all members of a species of plants, animals, or other organisms is referred to as extinction.
The primary cause of higher extinction rates is habitat loss. Other causes include habitat changes, commercial overexploitation of wildlife, the introduction of harmful nonnative species, pollution, and disease spread.
If a species is unable to adapt to changes in its ecosystem, it may relocate. The species may become threatened, endangered, or extinct if they do not move.
Thus, Extinction is likely if environmental conditions change faster than a species can evolve and members of that species lack the traits needed to survive in the new environment.
To learn more about the extinction, follow the link;
https://brainly.com/question/14480057
#SPJ6
What is the vapor pressure of water at 75 °C? mmHg (whole number)
What is the vapor pressure of bromine at 300 K? mmHg (whole number)
At what temperature is the vapor pressure of mercury 500 mmHg? °C (whole number)
What is the vapor pressure of diether ether at the normal freezing temperature of water? mmHg (whole number)
At what temperature will ethanol boil when at 50 mmHg? °C (whole number)
What is the normal boiling point pressure for water in kPa? kPa (exact number)
What is the normal boiling point pressure for water in mmHg? mmHg (exact number)
What is the normal boiling point temperature in Celsius of n-Octane? °C (whole number)
What is the normal boiling point temperature in Kelvin of Ethylene glycol? K (whole number)
At which temperature would ethylene glycol boil when the atmospheric pressure is 0.20 atm? °C (Whole number)
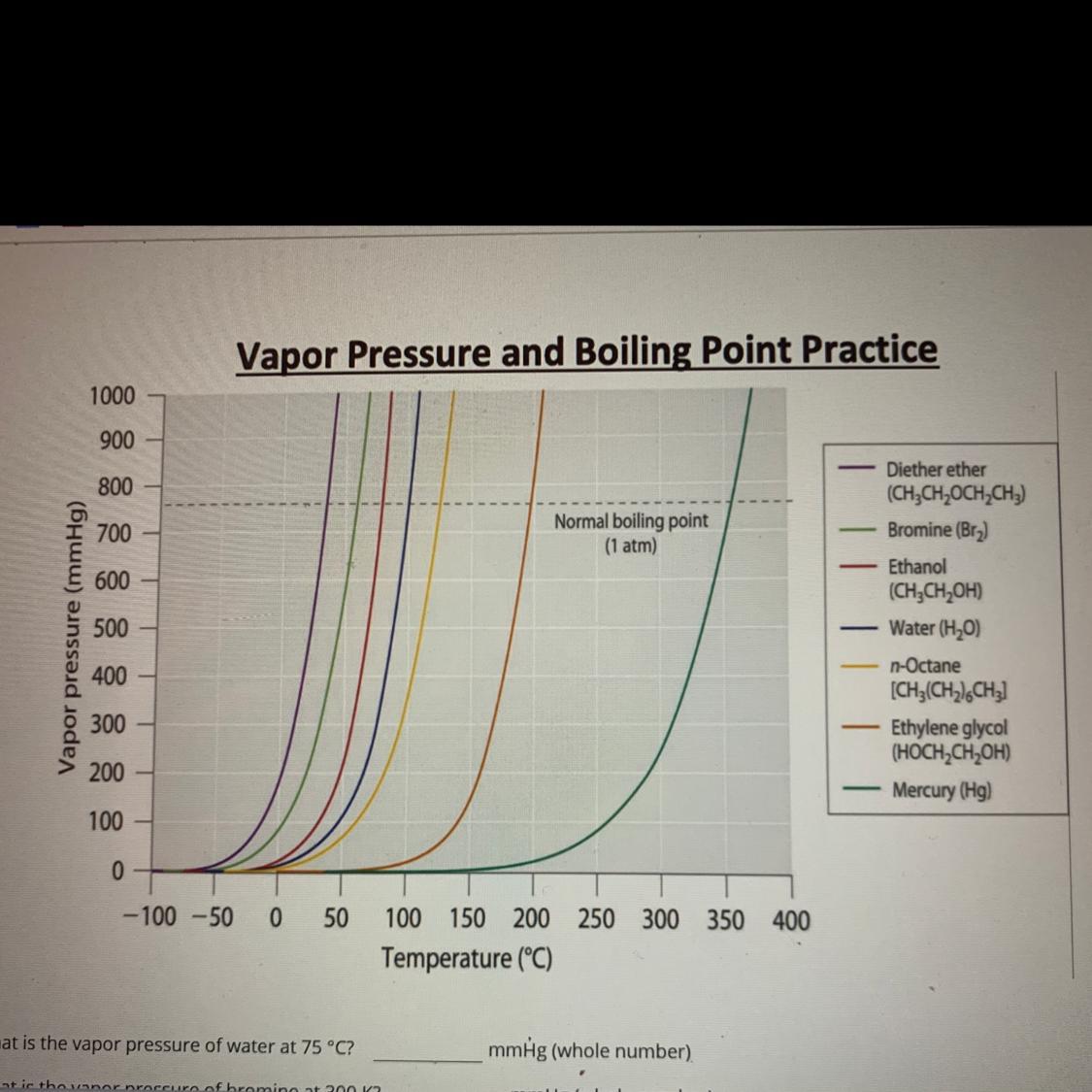
Answers
Answer: All the answers are given below.
Explanation:
The vapor pressure of water at 75°C is approximately 293 mmHg (whole number).
The vapor pressure of bromine at 300 K is approximately 240 mmHg (whole number).
The boiling point of mercury is 357°C at atmospheric pressure (760 mmHg), and the vapor pressure of mercury is 500 mmHg at a higher temperature than this. Therefore, the temperature at which the vapor pressure of mercury is 500 mmHg is greater than 357°C.
Diethyl ether's normal boiling point is 34.6°C, which is above the freezing temperature of water (0°C). At 0°C, the vapor pressure of diethyl ether is approximately 5.5 mmHg (whole number).
At a pressure of 50 mmHg, ethanol will boil at approximately 64°C (whole number).
The normal boiling point pressure for water is 101.3 kPa (exact number) at a temperature of 100°C.
The normal boiling point pressure for water is 760 mmHg (exact number) at a temperature of 100°C.
The normal boiling point temperature in Celsius of n-Octane is approximately 126°C (whole number).
The normal boiling point temperature in Kelvin of ethylene glycol is approximately 471 K (whole number).
To find the boiling point of ethylene glycol at a pressure of 0.20 atm, you can use the Clausius-Clapeyron equation. However, the equation requires knowing the vapor pressure of ethylene glycol at a known temperature. Without this information, it is not possible to calculate the boiling point.
When a small piece of copper metal is added to a silver nitrate solution, the following reaction occurs: 2Ag+NO3+Cu → Cu (NO3)2+2Ag
This equation represents both a single replacement reaction AND a(n) ______________________ reaction.
Question 4 options:
A. oxidation - reduction
B. neutralization
C. combustion
D. decomposition
Answers
Answer:
A. Oxidation-reduction
Explanation:
I assume you mean the reaction is:
Ag2NO3(aq) + Cu(s) -> 2Ag(s) + Cu(NO3)2(aq)
Either way:
Solids have the oxidation number of 0. So in the beginning of the reaction Cu(s) has the oxidation number 0, and at the end it has a oxidation number of +2. So it was oxidized.
Ag in the beginning of the reaction has the oxidation number of +1, and ends with the oxidation number of 0. It was reduced.
So its an oxidation reduction.
What energy is associated with a photon in the infrared region of the spectrum having a frequency of 2.9 × 1013 5-1?
Answers
Answer:
1.9 x 10^-20 Joules
Explanation:
I'll assume the frequency is 2.9 x 10^13 Hz [5-1 was meant to be s^-1].
The energy of a photon is given by the releationship:
E = hf, where E is energy, h is Planck's constant, and f is frequency, in 1/s or Hz.
Planck's constant = 6.62607E-34 J*s
E = hf
E = (6.626 x 10^-34 J*s)*(2.9 x 10^13 s^-1)
E = 1.9 x 10^-20 Joules
where is the chemical energy stored in adenosine triphosphate (atp), as shown below?
Answers
Part A Which wavelength of light (in nanometers) is emitted if an electron moves from the conduction band to the valence band in a sample of silicon? (Silicon has a band gap of 1.1 eV.) Express your answer to two significant figures and include the appropriate units.
Answers
Part A: The wavelength of light emitted when an electron moves from the conduction band to the valence band in silicon is approximately 1127 nm (nanometers).
To calculate the wavelength of light emitted, we will use the energy gap and the following formula:
Wavelength (λ) = (hc) / E
Where:
- λ is the wavelength
- h is the Planck's constant (6.63 x 10⁻³⁴ Js)
- c is the speed of light (3 x 10⁸m/s)
- E is the energy gap (in Joules)
First, we need to convert the energy gap from eV (electron volts) to Joules:
1.1 eV * (1.6 x 10⁻¹⁹ J/eV) = 1.76 x 10⁻¹⁹ J
Now, we can calculate the wavelength:
λ = (6.63 x 10⁻³⁴ Js * 3 x 10⁸ m/s) / (1.76 x 10⁻¹⁹ J)
λ = 1.127 x 10⁻⁶ m
To express the wavelength in nanometers and two significant figures, we have:
λ = 1127 nm
To know more about conduction band click on below link:
https://brainly.com/question/30890306#
#SPJ11
Thorium 238 Th produces a daughter nucleus that is radioactive. The daughter, in turn, produces its own radioactive daughter, and so on. This process continues until bismuth 283 Bi is reached. What are (a) the total number Na of a particles and (b) the total number Ne of ßparticles that are generated in this series of radioactive decays
Answers
(a) The total number of alpha particles (a) generated in the Thorium-238 to Bismuth-283 decay series is 13.
(b) The total number of beta particles (ß) generated in the decay series is 22.
To determine the total number of alpha particles (a) and beta particles (ß) generated in the radioactive decay series from Thorium-238 (238 Th) to Bismuth-283 (283 Bi), we need to examine the decay steps and track the particles emitted at each step.
The decay series is as follows:
238 Th -> 234 Pa -> 234 U -> 230 Th -> 226 Ra -> 222 Rn -> 218 Po -> 214 Pb -> 214 Bi -> 214 Po -> 210 Pb -> 210 Bi -> 210 Po -> 206 Pb -> 206 Bi -> 206 Po -> 202 Tl -> 202 Pb -> 202 Bi -> 202 Po -> 198 Pb -> 198 Bi -> 198 Po -> 194 Pb -> 194 Bi -> 194 Po -> 190 Pb -> 190 Bi -> 190 Po -> 186 Pb -> 186 Bi -> 186 Po -> 182 Hg -> 182 Tl -> 182 Pb -> 182 Bi -> 182 Po -> 178 Pb -> 178 Bi -> 178 Po -> 174 Pb -> 174 Bi -> 174 Po -> 170 Pb -> 170 Bi -> 170 Po -> 166 Pb -> 166 Bi -> 166 Po -> 162 Tl -> 162 Pb -> 162 Bi -> 162 Po -> 158 Pb -> 158 Bi -> 158 Po -> 154 Pb -> 154 Bi -> 154 Po -> 150 Pb -> 150 Bi -> 150 Po -> 146 Pb -> 146 Bi -> 146 Po -> 142 Pb -> 142 Bi -> 142 Po -> 138 Pb -> 138 Bi -> 138 Po -> 134 Te -> 134 Sb -> 134 Sn -> 134 In -> 134 Cd -> 134 Ag -> 134 Pd -> 134 Rh -> 134 Ru -> 134 Tc -> 134 Mo -> 134 Nb -> 134 Zr -> 134 Y -> 134 Sr -> 134 Rb -> 134 Kr -> 134 Br -> 134 Se -> 134 As -> 134 Ge -> 134 Ga -> 134 Zn -> 134 Cu -> 134 Ni -> 134 Co -> 134 Fe -> 134 Mn -> 134 Cr -> 134 V -> 134 Ti -> 134 Sc -> 134 Ca -> 134 K -> 134 Ar -> 134 Cl -> 134 S -> 134 P -> 134 Si -> 134 Al -> 134 Mg -> 134 Na -> 134 Ne -> 283 Bi
(a) To find the total number of alpha particles (a) generated, we need to count the number of alpha decays in the series. Each decay results in the emission of one alpha particle. By counting the number of steps that involve alpha decay, we can determine the total number of alpha particles produced.
Counting the steps, we find that there are 13 alpha decays in the series.
Therefore, the total number of alpha particles (Na) generated in this series of radioactive decays is 13.
(b) To find the total number of beta particles (ß) generated, we need to count the number of beta decays in the series. Each beta decay involves the emission of one beta particle. By counting the number of steps that involve beta decay, we can determine the total number of beta particles produced.
Counting the steps, we find that there are 22 beta decays in the series.
Therefore, the total number of beta particles (Ne) generated in this series of radioactive decays is 22.
Read more on decay series here: https://brainly.com/question/16355768
#SPJ11