Which element in the periodic table would be the mot likely to gain electron in a chemical bond
Answers
The right-hand side of the atomic numbers contains nonmetals, which have relatively high electronegativity values and a propensity to gain electrons.
What is chemical bond explain?Atoms in molecules are held together by chemical bonds. Electrostatic forces between negative-charged electrons and ionized atomic nuclei produce bonds (the positions of which in space are determined by quantum mechanics).
How does a chemical bond develop?A chemical bond is the term used to describe the attraction amongst atomic nuclei. To form bonds, atoms exchange or swap their valence electrons. The valence electrons, which make up an atom's lowest energy level, have the capacity to interact chemically. The most straightforward explanation is that atoms are trying to enter the safest (lowest-energy) state they can.
To know more about Chemical bond visit:
https://brainly.com/question/15444131
#SPJ4
Related Questions
Which of the following processes is NOT a way that carbon could move between the atmosphere and the biosphere?
Question 4 options:
respiration (breathing)
combustion (burning)
deep burial, compaction, and cementation
photosynthesis
Question 5 (2 points)
What is the only natural way that carbon can move OUT of the geosphere?
Question 5 options:
burning of fossil fuels
volcanic eruptions
dissolution
decomposition
Question 6 (1 point)
Which carbon reservoir contains the MOST carbon?
Question 6 options:
fossil fuels
atmosphere
land biomass
rocks
Question 7 (2 points)
Which carbon reservoir changes the quickest and has the biggest direct effect on climate?
Question 7 options:
land biomass
ocean
atmosphere
rocks
Question 8 (2 points)
Which best describes the process of ocean acidification?
Question 8 options:
The ocean absorbs more human-produced CO2, causing the acidity to increase
The ocean releases more human-produced CO2, causing the acidity to decrease
The ocean absorbs more natural CO2, causing the acidity to decrease
The ocean receives more polluted runoff, causing the acidity to increase
Question 9 (2 points)
What is a potential impact of ocean acidification?
Question 9 options:
Animals will have a harder time building their shells
Corals may have a harder time building their skeletons
Ocean ecosystems may suffer, making it harder for humans to get food from the ocean
All of the above
Answers
Answer:
question 1 i believe is c i will put the other answeres in comments
when i finish the test
Explanation:
The carbon cycle plays an important in maintaining carbon balance on the earth.
What is the carbon cycle?The carbon cycle is the cycle by which carbon is recycled between the atmosphere and the geosphere.
In the carbon cycle, the process of recycling carbon do not include deep burial, compaction, and cementation.
Volcanic eruptions are one natural way that carbon can move OUT of the geosphere.
Fossil fuels, atmosphere, land biomass and rocks are all carbon reservoirs, but fossil fuels contains the MOST carbon.
The atmosphere is the carbon reservoir that changes the quickest and has the biggest direct effect on climate.
Ocean acidification occurs because the ocean absorbs more human-produced CO2, causing the acidity to increase.
A potential impact of ocean acidification is that ocean ecosystems may suffer, making it harder for humans to get food from the ocean.
Therefore, the carbon cycle is important in maintaining carbon balance on the earth.
Learn more about carbon cycle at: https://brainly.com/question/25845923
What is an addition reaction of alkenes ?
Answers
\(\huge\pink{\boxed{\tt{\colorbox{pink}{ANSWER}}}}\)
Chemical transformation of a carbon-carbon double bond is the addition reaction.
Explanation:
Consequently, if the bond energies of the product molecules are greater than the bond energies of the reactants, the reaction will be exothermic.
hope it helps
which set of elements is arranged in order of increasing electronegativity?which set of elements is arranged in order of increasing electronegativity? p < f < si < s s < f < p < si f < s < p < si si < p < s < f
Answers
The correct order of increasing electronegativity for the given set of elements is: s, p, si, f, where fluorine (F) has the highest electronegativity and sodium (Na) is not included in the set.
Electronegativity is a property that describes the tendency of an atom to attract electrons towards itself when it is part of a chemical bond. In general, electronegativity increases across a period from left to right and decreases down a group in the periodic table.
Looking at the given sets of elements: p, f, si, s and s, f, p, si, we can determine the order of increasing electronegativity.
The correct set of elements arranged in order of increasing electronegativity is: s, p, si, f.
Fluorine (F) is the most electronegative element in the periodic table, and it attracts electrons strongly due to its small atomic size and high effective nuclear charge. Therefore, it has the highest electronegativity.
Following fluorine, oxygen (O) has a higher electronegativity than sulfur (S), which in turn has a higher electronegativity than phosphorus (P). This is because electronegativity generally increases across a period from left to right.
Silicon (Si) is less electronegative than phosphorus but more electronegative than sulfur. It is positioned in the middle of the order.
Lastly, sodium (Na) is less electronegative than silicon and is not included in the given set.
(Note: The set s, f, p, si is not in the correct order of increasing electronegativity, as fluorine should have the highest electronegativity.)
For more such information on: electronegativity
https://brainly.com/question/18258838
#SPJ8
A feasible solution is one that satisfies at least one of the constraints in the problem. a. true b. false
Answers
b) FALSE A feasible solution must attempt to satisfy all the major constraints in the problem.
What is feasible solution?A basic viable solution in the theory of linear programming is one that has a small number of non-zero variables. Each viable solution polyhedron's corner correlates geometrically to a BFS. There must be an optimum BFS if there is an optimal solution.
to learn more about feasible solution go to -
https://brainly.com/question/15398513
#SPJ4
What is the electron configuration for a neutral atom in the ground state with a total of 6 valence electrons?
Answers
The electron configuration for a neutral atom in the ground state with a total of 6 valence electrons is 1s²2s²2p⁴, option A.
The distribution of electrons in an element's atomic orbitals is described by the element's electron configuration.
Atomic electron configurations adhere to a standard nomenclature in which all atomic subshells that contain electrons are arranged in a sequence with the number of electrons they each possess expressed in superscript. For instance, sodium's electron configuration is 1s22s22p63s1.
For a neutral atom such as oxygen , whose atomic number is 8 , has 8 electrons .2 electrons are present in its 1st shell and 6 valence electrons are present in its outermost shell or 2nd shell , such that the electronic configuration of the atom becomes 1s²2s²2p⁴.
The arrangement of an atom's or molecule's electrons in atomic or molecular orbitals is known as the electron configuration in atomic physics and quantum chemistry.
Learn more about Electronic configuration:
https://brainly.com/question/28534782
#SPJ4
Complete question:
Which is the electron configuration of a neutral atom in the ground state with a total of six valence electrons?
1) 1s^2 2s^2 2p^4
2) 1s^2 2s^2 2p^6
3) 1s^2 2s^2 2p^2
4) 1s^2 2s^2 2p^6 3s^2 3p^6
the next questions are related to the titration of 30.00 ml of a 0.0700 m acetic acid solution with 0.0900 m koh. what is the initial ph of the analyte solution?
Answers
The initial pH of the analyte solution can be determined using the Henderson-Hasselbalch equation, which relates the pH of a solution to the pKa and the ratio of the concentrations of the acid and its conjugate base.
The pKa of acetic acid is 4.76. The initial concentration of acetic acid is 0.0700 M, and the concentration of its conjugate base (acetate ion) can be calculated from the stoichiometry of the reaction (1:1) and the volume and concentration of the KOH solution used in the titration. Once the concentrations of the acid and its conjugate base are known, the pH can be calculated using the Henderson-Hasselbalch equation. The initial pH of the analyte solution is 4.74. To determine the initial pH of the 30.00 mL, 0.0700 M acetic acid solution (analyte solution) before titration with 0.0900 M KOH, we can use the Ka expression for weak acids. Acetic acid (CH₃COOH) is a weak acid with a Ka value of 1.8 x 10⁻⁵. By setting up an ICE table (Initial, Change, Equilibrium) and solving for the hydrogen ion concentration [H⁺], we can find the initial pH.
In this case, initial [CH₃COOH] = 0.0700 M, [H⁺] = 0, and [CH₃COO⁻] = 0. After calculating the equilibrium concentrations and substituting them into the Ka expression, we can find the [H⁺]. Finally, use the pH formula, pH = -log[H⁺], to calculate the initial pH of the analyte solution.
To know about analyte :
https://brainly.com/question/29804070
#SPJ11
According to a recent pol, 25% of adults in a certain area have high levels of cholesterol. They ceport that such elevated fevels "could be financialy devastating to the regions heathcare instem" and are a major concern to health insurance providers. Assume the standard deviation from the recent studies is accurate and known. According to recent studies, cholesterol levels in healthy adults from the area average about 205 mg/dL, with a standard deviation of about 35 mg/dL, and are roughly Normally distributed. If the cholesterol levels of a sample of 46 healthy adults from the region is taken, answer parts (a) through (d)
(a) What is the probability that the mean cholesterol level of the sample will be no more than 205?
Plys 205) 0.5 (Round to three decimal places as needed.)
(b) What is the probability that the mean cholesterol level of the sample will be between 200 and 2107
P(200
(c) What is the probability that the mean cholesterol level of the sample will be less than 1957
Ply<195) (Round to three decimal places as needed)
(d) What is the probability that the mean cholesterol level of the sample will be greater than 2179
Py>217) (Round to three decimal places as needed)
Answers
Hence, the probability that the mean cholesterol level of the sample will be greater than 217 is 0.034. Answer: 0.034.According to the given statement, we have the following data.
mean (μ) = 205 mg/dLstandard deviation
(σ) = 35 mg/dLsample size
(n) = 46(a) Probability that the mean cholesterol level of the sample will be no more than 205.To find this, we will use the z-score formula.z
= (x - μ) / (σ/√n)Here,
x = 205
μ = 205
σ =
35n
= 46Plugging in these values, we get,
z = (205 - 205) / (35/√46)
z = 0Hence, the probability that the mean cholesterol level of the sample will be no more than 205 is 0.5. Answer: 0.5
(b) Probability that the mean cholesterol level of the sample will be between 200 and 210:
To find this, we need to standardize the values and use the z-table.P(z < (210 - 205) / (35/√46)) - P(z < (200 - 205) / (35/√46))P(z < 1.65) - P(z < -1.65) = 0.4495 - 0.0505
= 0.3990Hence, the probability that the mean cholesterol level of the sample will be between 200 and 210 is 0.3990. Answer: 0.3990
(c) Probability that the mean cholesterol level of the sample will be less than 195: To find this, we need to standardize the values and use the z-table.P(z < (195 - 205) / (35/√46))P(z < -2.91) = 0.002Hence, the probability that the mean cholesterol level of the sample will be less than 195 is 0.002. Answer: 0.002
(d) Probability that the mean cholesterol level of the sample will be greater than 217: To find this, we need to standardize the values and use the z-table.P(z > (217 - 205) / (35/√46))P(z > 1.82) = 0.034 Answer: 0.034.
To know more about data visit:
https://brainly.com/question/29117029
#SPJ11
What are the answers to these?

Answers
Answer:
1)physical change
Explanation:
2)chemical change
POSSIBLE POINT
An engine cylinder contains 250.0 mL of gas at a pressure of 1.00 atm. As the engine runs, it compresses the cylinder, reducing the volume of the
25.0 mL. What is the new pressure of the gas at this volume?
Answers
Answer: The new pressure of the gas at this volume is 10 atm.
Explanation:
Given: \(V_{1}\) = 250 mL, \(P_{1}\) = 1.00 atm
\(V_{2}\) = 25.0 mL, \(P_{2}\) = ?
According to Boyle's law, at constant temperature the pressure of an ideal gas is inversely proportional to volume.
Therefore, formula used is as follows.
\(P_{1}V_{1} = P_{2}V_{2}\)
Substitute the values into above formula as follows.
\(P_{1}V_{1} = P_{2}V_{2}\\1.00 atm \times 250.0 mL = P_{2} \times 25.0 mL\\P_{2} = 10 atm\)
Thus, we can conclude that the new pressure of the gas at this volume is 10 atm.
which product would be formed when water, in the presence of sulfuric acid, is added to 2-methyl-2-pentene?
Answers
The product formed when water, in the presence of sulfuric acid, is added to 2-methyl-2-pentene would be 2-methyl-2-pentanol.
The sulfuric acid acts as a catalyst, allowing the water to add to the double bond of the 2-methyl-2-pentene molecule. This creates a hydroxyl group, -OH, on each of the two carbon atoms in the double bond and forms a new molecule of 2-methyl-2-pentanol. This reaction is a type of hydration reaction, which is a reaction between an alkene and water, and it is an example of an addition reaction. The reaction is exothermic, meaning it releases heat and creates a hydroxyl group, which is an alcohol-functional group. This reaction is also reversible, meaning that it can be reversed by adding heat.
To know more about sulfuric acid refer to the link brainly.com/question/29303579
#SPJ4
Which one of the following is a measure of the certainty of a
result comparing to a given value or the agreement of
measurements?
a. Accuracy
b. precision
c. Percentage
d. Uncertainty
Answers
Answer: a. Accuracy how close is your measurement tot the true value
Explanation:
Which one of the following is a measure of the certainty of a
result comparing to a given value or the agreement of
measurements?
a. Accuracy how close is your measurement tot the true value
b. precision; how close are yourr measurements to each other
c. Percentage: the ratio of tour measurement to the true value X 100
d. Uncertainty: how unsure are you of your measurements
For any substance, the melting point and freezing point are at the same temperature. TRUE OR FALSE
Answers
Answer:
This is false.
Explanation:
This is false because water freezes at 32 degrees faraheint and carbon would freeze at a different temperature.
what are valence electrons? why are they so important in chemistry?
Answers
Answer:
Valence electrons are negatively charged particles located in the outermost shell of atoms that can be transferred or shared with other atoms. Valence electrons are important in chemistry because the number of valence electrons in a particular atom can be used to determine how the atom will react chemically with other atoms.
Gravity is a (n) _____________ between objects and depends on an object's size and their distance apart.
Answers
Gravity is a (n) force between objects and depends on an object's size and their distance apart.
Gravitational force is the attractive that exist between all object with mass an object with mass attracts another object with mass the magnitude of the force is directly proportional to the masses of the two objects and inversely proportional to the square of the distance between the two objects
Know more about objects
https://brainly.com/question/27786016
#SPJ1
What is the total number of pairs of electrons that one carbon atom shares?
Answers
Answer:
4
Explanation:
A carbon atom has 4 electrons in its outermost shell (2s^2p^2). All are unpaired (none share their orbital with another electron). So all four are anxious to pair with another electron. Once it has found 4 more electrons contributed from other atom(s), it will have 4 pairs of shared electrons.
Hydrogen has one lone electron. An atom of H is downright gleeful in sharing it's electron with elements such as carbon, C. Since carbon has 4 unpaied electrons, it will combine with 4 H atoms. At that point, cabon is sharing 4 electron pairs.
What is the % of each element in Ni3{PO4) 2 ?
Answers
Answer:
nickel 48.1063%
Phosphorus 16.9245%
Oxygen 34.9692%
The percent composition of each element like nitrogen, oxygen and phosphorous in Ni₃(PO₄)₂ are 48.03%, 34.97% and 16.93% respectively.
How do we calculate % composition?Percent composition of any element present in any compound will be calculated as:
% comosition = (Mass of element / Mass of compound)×100%
Mass of Ni₃(PO₄)₂ compound = 366.02 g/mol
Molar mass of 3 Nitrogen atoms = 3×58.6 = 175.8 g/mol
Moar mass of 2 Phosphorous atoms = 2×31 = 62 g/mol
Moar mass of 8 Oxygen atoms = 8×16 = 128 g/mol
% comosition of Nitrogen = (175.8/366.02)×100% = 48.03%
% comosition of Oxygen = (128/366.02)×100% = 34.97%
% comosition of Phosphorous = (62/366.02)×100% = 16.93%
Hence % composition of nitrogen, oxygen and phosphorous is 48.03%, 34.97% and 16.93% respectively.
To know more about percent composition, visit the below link:
https://brainly.com/question/21044245
What is the frequency of a red laser that has a wavelength of 676 mn
Answers
The frequency of a red laser that has a wavelength of 676 nm would be 4.43 x \(10^{14\) hertz.
Frequency of wavesThe frequency and wavelength of a wave are related by the following equation:
λf = c
Where λ is the wavelength of the wave in meters, f is the frequency in Hertz, and c is the speed of light in a vacuum.
in this case, λ = 676 nm = 6.76 x \(10^{-7\) m
c = 299,792,458 m/s
Making f the subject of the formula:
f = c/λ
= 299,792,458/6.76 x \(10^{-7\)
= 4.43 x \(10^{14\) hertz
In other words, the frequency of a red laser that has a wavelength of 676 nm would be 4.43 x \(10^{14\) hertz.
More on waves can be found here: https://brainly.com/question/29334933
#SPJ1
Question 16 Identify the statement that is true about nonelectrolytes Most nonelectrolytes are ionic compounds_ Nonelectrolytes dissolve in water to produce ions. Nonelectrolytes do not dissociate in water: Nonelectrolytes conduct electricity:
Answers
The statement that is true about nonelectrolytes is that nonelectrolytes do not dissociate in water. This means that they do not produce ions when dissolved in water.
What are nonelectrolytes?Nonelectrolytes are compounds that do not conduct electricity when dissolved in water. They are also unable to form ions in a solution. Ethanol, glucose, and sucrose are examples of nonelectrolytes. Electrolytes are chemical compounds that can conduct electricity when dissolved in water. Electrolytes are divided into two types, strong and weak electrolytes. NaCl and HCl are examples of strong electrolytes, while CH₃COOH and NH₄OH are examples of weak electrolytes.
Nonelectrolytes do not dissociate in water, unlike electrolytes, which break up into cations and anions when dissolved in water. When an electric potential is placed across an electrolyte, these ions transport electrical charge, whereas nonelectrolytes do not.
Among the given options, "Nonelectrolytes do not dissociate in water" is the statement that is true about nonelectrolytes.
Learn more about Nonelectrolytes here: https://brainly.com/question/23251215
#SPJ11
Describe the delocalization of pi (π) electrons and explain how this can account for the structure and stability of the carbonate ion, CO32-
Answers
Delocalization of pi (π) electrons refers to the spreading of electrons over a larger region than would be expected in a localized bond. The carbonate ion, CO32-, exhibits delocalization of its pi electrons, which can account for its structure and stability.
In the carbonate ion, the three oxygen atoms are bonded to the central carbon atom in a planar arrangement. The double bonds between the carbon and oxygen atoms consist of both sigma (σ) and pi (π) bonds. The pi electrons are delocalized over the three oxygen atoms, resulting in the formation of a resonating structure.
This delocalization of pi electrons contributes to the stability of the carbonate ion by reducing the repulsive forces between the negatively charged oxygen atoms. The delocalization of the pi electrons spreads the negative charge over a larger region, reducing the strength of the repulsive forces between the oxygen atoms and allowing the carbonate ion to maintain its planar structure.
In addition, the delocalization of the pi electrons increases the electron density around the central carbon atom, making it more difficult for the carbonate ion to react with other species and increasing its stability.
In summary, the delocalization of pi electrons in the carbonate ion, CO32-, contributes to its stability by reducing the repulsive forces between the negatively charged oxygen atoms and increasing the electron density around the central carbon atom. This delocalization of pi electrons plays a key role in determining the structure and stability of the carbonate ion.
Learn more about resonating structure here:
https://brainly.com/question/29547999
#SPJ4
The most important element on earth Is Oxygen O water a hydrogen () carbon
Answers
Answer:
Carbon
Explanation:
Carbon is the most important element to life. Without this element, life as we know it would not exist. As you will see, carbon is the central element in compounds necessary for life.
Answer:
Carbon is the most important element
In a solution containing 21.2 g sodium carbonate in 1.50 L of solution, calculate
the concentration of the sodium and carbonate ions.
Answers
Answer:
0.13 molL-1
Explanation:
We must first obtain the number of moles of sodium carbonate as follows;
Number of moles = mass/molar mass
Number of moles = 21.2 g/106 g/mol
Number of moles= 0.2 moles
Number of moles = concentration × volume
Volume = 1.50 L
Concentration = number of moles/volume
Concentration = 0.2 moles/1.5 L
Concentration = 0.13 molL-1
Naturally occurring magnesium exists as three isotopes. 78.70% is Mg-24 with a mass of 23.98504 amu, 10.13% is Mg-25 with a mass of 24.98584 amu, and 11.17% is Mg-26 with a mass of 25.98259 amu. What is the atomic mass of magnesium?
Answers
Answer:
The correct answer is 24.31 amu.
Explanation:
The formula for finding the average atomic mass = The sum of the percentage abundance of isotopes × the actual mass of isotopes
The average atomic mass = % abundance of Mg-24 × actual mass + % abundance of Mg-25 × its actual mass + % abundance of Mg-26 × its actual mass
Avg. Atomic mass = 0.7870 × 23.98504 + 0.1013 × 24.98584 + 0.1117 × 25.98259
Avg. atomic mass = 18.88 + 2.53 + 2.90 = 24.31 amu
The atomic mass of magnesium is 24.31 amu.
Write a balanced chemical equation for the following reactions:
1) Calcium hydroxide+ Carbon dioxide → Calcium carbonate + water
Answers
Ca(OH)
2
+CO
2
⟶CaCO
3
+H
2
O
What volume of a 3.5 M HCI is required to completely neutralize a 50.0 ml of a 2.0 M NaOH
Answers
The volume of the 3.5 M HCI that is required to completely neutralize the 50.0 ml of the 2.0 M NaOH is 28.57 mL.
The molarity of the HCl, M₁ = 3.5 M
The volume of the HCl, V₁ = ?
The molarity of the NaOH, M₂ = 2.0 M
The volume of the NaOH, V₂ = 50 mL
To neutralize the reaction , the volume of the HCl required is as :
M₁ V₁ = M₂ V₂
Where,
M₁ = 3.5 M
V₁ = ?
M₂ = 2 M
V₂ = 50 mL
( 3.5 × V₁ ) = ( 2 × 50 )
3.5 V₁ = 100
V₁ = 28.57 mL
The volume of the HCl required to completely neutralize is the 28.57 mL with the molarity of the HCl is the 3.5 M.
To learn more about molarity here
https://brainly.com/question/14025987
#SPJ1
Why are incandescent light bulbs considered to be lower efficiency than LED bulbs?
#1: A greater percentage of the energy output of the incandescent bulb goes toward heat.
#2: A greater amount of energy is required for the same light output from the LED bulb.
#3: A smaller amount of energy is wasted by the incandescent bulb.
#4: A smaller percentage of the energy output of the LED bulb goes toward light.
Answers
A smaller amount of energy is wasted by the incandescent bulb.
Why are incandescent light bulbs less efficient?Because so much energy (90%) is used to produce heat instead of light, incandescent lights are the least efficient of all current lighting options.A typical incandescent source emits around 2% of its energy as usable visible light and the rest 98% as waste heat. Incandescent bulbs have a high likelihood of burning out regularly and need to be replaced annually.Incandescent bulbs are inefficient since they only produce 10% of light and 90% of heat. Additionally, incandescent lights don't last as long as CFLs and LEDs do. A 12-watt LED bulb lasts 25,000 hours, compared to 1,000 hours for an incandescent bulb, 10,000 hours for a CFL, and 15 hours for a CFL.To learn more about incandescent light bulbs refers to:
brainly.com/question/8979272
#SPJ1
describe nucleus of atom
Answers
Answer:
the nucleus is made up of protons and neutrons
Predict the shape of the molecule.
B. trigonal planar
A. linear
D. bent
C. tetrahedra
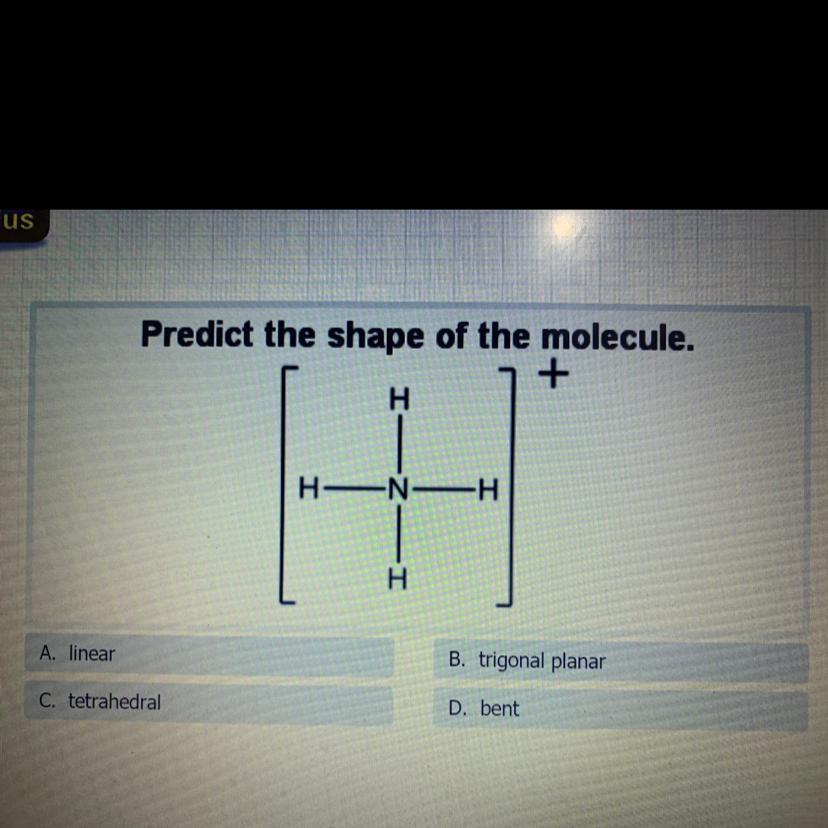
Answers
Can you find if a substance is either pure k_2o or pure k_2o_2 when all you have is the mass of k?
Answers
Yes, the chemist can identify the substance in the sample.
When describing a compound's mass per mole, the term "molar mass" is employed. The molar mass is determined by multiplying the individual masses of all the atoms that make up a compound.
K₂O has a molar mass of 94.2 g/mol.
has a molar mass of 110.196 g/mol.
By multiplying the masses of each individual atom—one K atom weighs 39.0983 g/mol, whereas one O atom weighs 15.999 g/mol—the molar masses are obtained.
When the mass of a pure compound is known, the mass of oxygen (O) can be calculated by subtracting the mass of potassium (K) from the total mass. Since each of the mentioned compounds contain two K atoms, the difference in oxygen mass can be utilised to identify the pure chemical.
For instance, after deducting the mass of the K from the unknown molecule, the residual mass will be approximately 16 g if the compound is K₂O and approximately 32 g if the compound is K2O2.
To know about mass
https://brainly.com/question/15631880
#SPJ4
The complete question is
The compound K2O2 exists. A chemist can determine the mass of K in a sample of known mass that consists of either pure K₂O or pure K2O2. From this information, can the chemist answer the question of which compound is in the sample? Explain
if a substance x has a solubility of 2.4×10−5 mg/l, and a molar mass of 188 g/mol, what is the molar solubility of the substance?
Answers
To find the molar solubility of substance X, we need to convert the given solubility from milligrams per liter (mg/L) to moles per liter (mol/L). The molar solubility of substance X is 1.28 × 10^(-10) mol/L.
Step 1:
We need to convert 2.4×10−5 mg/L to grams per liter (g/L) by dividing by 1000, which gives us 2.4×10−8 g/L.
Solubility = 2.4 × 10^(-5) mg/L
1 mg = 0.001 g, so:
Solubility = 2.4 × 10^(-5) × 0.001 g/L = 2.4 × 10^(-8) g/L
Step 2:
Next, we need to convert the mass to moles using the molar mass of substance X.
Molar mass = 188 g/mol
Molar solubility =2.4×10−8 g/L ÷ 188 g/mol = 1.28×10−10 mol/L
This is the molar solubility of substance X.
The molar solubility of substance X is 1.28×10−10 mol/L.
To know more about solubility visit:
brainly.com/question/31493083
#SPJ11
Which circuit hook-up design will have the brightest light bulb?A - 1 battery 1 bulb, B - 2 batteries 1 bulb, C - 3 batteries one bulb, D 2 batteries the bulb is on the other end, E 1 battery and 1 bulb on the other end
Question 5 options:
A
B
C
D
E
Heres another for you guys!

Answers
Answer:
I believe it is E because the battery has a wire, a power source(the battery), and a bulb connected together thus making a full series circuit. Furthermore, the more batteries they are to a series circuit, the more dimmer the light bulbs will be.