When we react a weak acid with a strong base of equal amounts and concentration, the component of the reaction that will have the greatest effect on the pH of the solution is: Select the correct answer below:_________
a. the reactant acid
b. the reactant base
c. the conjugate acid of the strong base
d. the conjugate base of the weak acid
Answers
Answer:
d. the conjugate base of the weak acid
Explanation:
The strong base (BOH) is completely dissociated in water:
BOH → B⁺ + OH⁻
The resulting conjugate acid (OH⁻) is a weak acid, so it remains in solution as OH⁻ ions.
By other hand, the weak acid (HA) is only slightly dissociated in water:
HA ⇄ H⁺ + A⁻
The resulting conjugate base (A⁻) is a weak base. Thus, it reacts with H⁺ ions from water to form HA, increasing the concentration of OH⁻ ions in the solution.
Therefore, the resulting solution will have a pH > 7 (basic).
Related Questions
What is the mass of 1.78 moles of O2
Answers
Answer:
56.96 grams
Explanation:
To find the mass of 1.78 moles of O2, we need to use the molar mass of O2, which is the mass of one mole of O2.
The chemical formula for O2 is O-O or simply O2. The molar mass of O2 is the sum of the atomic masses of two oxygen atoms, which can be found on the periodic table.
The atomic mass of oxygen (O) is approximately 16.00 g/mol. So the molar mass of O2 is:
Molar mass of O2 = 2 x atomic mass of O
= 2 x 16.00 g/mol
= 32.00 g/mol
Therefore, the mass of 1.78 moles of O2 is:
Mass = number of moles × molar mass
= 1.78 mol × 32.00 g/mol
= 56.96 g
So the mass of 1.78 moles of O2 is 56.96 grams.
Be sure to answer all parts.
A small hole in the wing of a space shuttle requires a 16.1 cm² patch.
(a) What is the patch's area in square kilometers (km²)? Enter your answer in scientific notation.
x 10
km²
(b) If the patching material costs NASA $2.94/in², what is the cost of the patch to the nearest cent?
Answers
A. The patch's area in square kilometers (km²) is 1.61×10⁻⁹ km²
B. The cost of the patch to the nearest cent is 734 cents
A. How to convert 16.1 cm² to square kilometers (km²)We can convert 16.1 cm² to km² as illustrated below:
Conversion scale
1 cm² = 1×10⁻¹⁰ km²
Therefore,
16.1 cm² = 16.1 × 1×10⁻¹⁰
16.1 cm² = 1.61×10⁻⁹ km²
Thus, 16.1 cm² is equivalent to 1.61×10⁻⁹ km²
B. How to determine the cost in centWe'll begin by converting 16.1 cm² to in². This can be obtained as illustrated below:
1 cm² = 0.155 in²
Therefore,
16.1 cm² = 16.1 × 0.155
16.1 cm² = 2.4955 in²
Finally, we shall the determine the cost in centas fo r llow:
Cost per in² = $2.94 = 294 centCost of 2.4955 in² =?1 in² = 294 cent
Therefore,
2.4955 in² = 2.4955 × 294
2.4955 in² = 734 cents
Thus, the cost of the patch is 734 cents
Learn more about conversion:
https://brainly.com/question/2139943
#SPJ1
MATCH THE NAMES OF THE MICROSCOPE PARTS WITH THEIR DECRIPTIONS
Answers
The Microscope part and their right descriptions are as follows
Iris Diaphragm: A. Increases or decreases the light intensity
Objective Lens System: B. After light passes through the specimen, it next enters this lens system
Stage: C. Platform that supports a microscope slide
Adjustment Knob: D. Causes stage (or objective lens) to move upward or downward
Condenser: E. Concentrates light onto the specimen
what other parts of microscope parts and their description should you know?Other parts of a microscope and their description that you should know about includes;
Eyepiece - The lens that you look through to see the image of the specimen.
Body tube - The tube that connects the eyepiece to the objective lenses.
Arm - The part of the microscope that supports the body tube and connects it to the base.
Base - The part of the microscope that supports the arm and provides stability.
Illuminator - The light source that provides light for the microscope.
Stage clips - The clips that hold the microscope slide in place on the stage.
Revolving nosepiece - The part of the microscope that holds the objective lenses and allows them to be rotated into place.
The above answer is in response to the full question below;
Match the names of the microscope parts in column A with the descriptions in column B. Place the letter of your choice in the space provided.
1. Iris diaphram
2. Objective lens system
3. Stage
4. Adjustment knob
5. Condenser
Increases or decreases the light intensity
2. After light passes through the specimen, it next enters this lens system
3. Platform that supports a microscope slide
4. Causes stage (or objective lens) to move upward or downward
5. Concentrates light onto the specimen
Find more exercises on Microscope;
https://brainly.com/question/1869322
#SPJ1
Write the net ionic equation for the reaction of magnesium metal with aqueous iron(II) nitrate. Include physical states.
Answers
The net ionic equation for the reaction of magnesium metal with aqueous iron(II) nitrate is as follows:
How to write net ionic equation?
Ionic equation is a chemical equation that show only ions that participate in a chemical reaction.
In other words, the ions that react together in solution and form new substances. The other ions that don't participate are called spectator ions.
According to this question, magnesium metal with aqueous iron(II) nitrate as follows:
2Mg(s) + 2Fe(NO₃)₂(aq) → 2Fe(s) + 2Mg(NO₃)₂(aq)
The net ionic equation for the reaction of magnesium metal with aqueous iron(II) nitrate is:
2 Fe²⁺ ---> 2 Fe(s) What is a net ionic equation?A net ionic equation is a chemical equation that shows only ions that participate in a chemical reaction. In other words, the ions react together in solution and form new substances.
The other ions that remain in the solution and do not form products are known as spectator ions.
The net ionic equation of the reaction of magnesium metal with aqueous iron(II) nitrate is given below as follows:
Molecular equation: 2 Mg (s) + 2 Fe (NO₃)₂(aq) → 2 Fe(s) + 2 Mg(NO₃)₂(aq)
Net ionic equation: 2 Fe²⁺ ---> 2 Fe(s)
Learn more about net ionic equations at: https://brainly.com/question/13879496
#SPJ1
2 SO2(g) + O2(g) + 2 H2O(ℓ) −→ 2 H2SO4(ℓ)
What mass in grams of SO2 is needed to
react with 1527 g of O2?
Answers
Answer:
6116g
Explanation:
2SO2(g) + O2(g) + 2H2O(ℓ) −→ 2H2SO4(ℓ)
We want to find the mass in grams of SO2 that is needed to react with 1527 g of O2. First we must convert the grams of O2 to moles of O2 then to moles of SO2 and then to grams of SO2
So first lets find the molar mass of O2
The mass of oxygen according to a periodic table is 15.999
Using this the mass of O2 would be 15.999(2) = 31.988g
Next we need to identify the mole ratio of O2 to SO2
Looking at the equation for 1 mole of O2 there are two moles of SO2
Next we need to find the molar mass of SO2
Again the mass of oxygen is 15.999g and the mass of Sulfur is 32.066
So the mass of SO2 would be 15.999(2) + 32.066 = 64.064g
Now that we have found all the needed conversions :
1 mol O2 = 31.988g 1 mol O2 = 2 mol SO21 mol SO2 = 64.064gWe can now use dimensional analysis to calculate the answer.
Kindly check the attached image to see the table. ( sorry if its a bit blurry )
Explanation : The conversions are used to cancel out the units to get to the final unit which is gSO2.
Once the units are cancelled out except for the gSO2 we mutliply and divide based off of what the table says to do.
Here first we divide 1527 by 31.988. We than multiply by 2. Finally we multiply by 64.064 to get the final answer which is 6116gSO2
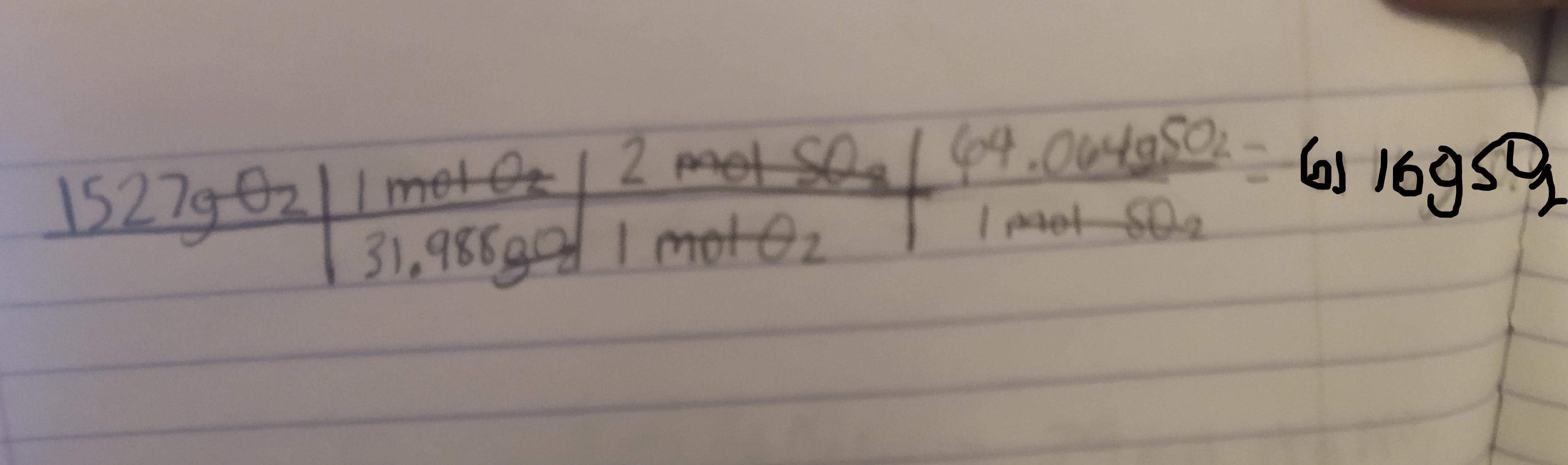
What is the compound name for magnesium sulfur

Answers
Answer:
MgS is the formula and Magnesium sulfide is compound name
Explanation:
Answer:
MgS
Explanation:
Calculate the pH of a [0.000765) M solution of KOH
Answers
Answer:
pH=10.88
Explanation:
Hello,
In this case, since potassium hydroxide is completely dissociated as shown below:
\(KOH\rightarrow K^++OH^-\)
For which we understand it is a base, more specifically, a strong base; it means that the concentration of the OH⁻ equals the concentration of the potassium hydroxide, that is 0.000765M, for that reason we can directly compute the pOH:
\(pOH=-log([OH^-])=-log(0.000765)=3.12\)
Finally, since the pOH and the pH are related by:
\(pOH+pH=14\)
The pH turns out:
\(pH=14-3.12\\pH=10.88\)
Best regards.
A 10.0 g gold ring with a specific heat 0.129 at 24.00°C is placed in a calorimeter with 118 g of water at 1.00°C.
What will be the final temperature of the system?
Answers
Answer:
1.06 °C
Explanation:
From the question given above, the following data were obtained:
Mass of gold (M₉) = 10 g
Specific heat capacity of gold (C₉) = 0.129 J/gºC
Initial temperature of gold (T₉) = 24 °C
Mass of water (Mᵥᵥ) = 118 g
Specific heat capacity of water (Cᵥᵥ) = 4.184 J/gºC
Initial temperature of water (Tᵥᵥ) = 1 °C
Equilibrium temperature (Tₑ) =?
The equilibrium temperature of the system can be obtained as follow:
Heat loss by the gold = heat gained by the water
M₉C₉(T₉ – Tₑ) = MᵥᵥCᵥᵥ(Tₑ – Cᵥᵥ)
10 × 0.129 (24 – Tₑ) = 118 × 4.184 (Tₑ – 1)
1.29(24 – Tₑ) = 493.712 (Tₑ – 1)
Clear bracket
30.96 – 1.29Tₑ = 493.712Tₑ – 493.712
Collect like terms
30.96 + 493.712 = 493.712Tₑ + 1.29Tₑ
524.672 = 495.002Tₑ
Divide both side by 495.002
Tₑ = 524.672 / 495.002
Tₑ = 1.06 °C
Therefore, the temperature of the system is 1.06 °C
The amount of heat of the system is measured by a device called a calorimeter. The final temperature of the system will be 1.06 degrees celsius.
What is equilibrium temperature?The equilibrium temperature is the temperature that follows the law of thermodynamics and is said to be the system that has alike temperatures.
Given,
Mass of Ag \(\rm (M_{g})\) = 10g
Specific heat capacity of Ag \((\rm C_{g})\) = \(\rm 0.129 J/g^{\circ}C\)
The initial temperature of Ag \((\rm T_{g})\) = \(24 ^{\circ}\;\rm C\)
Mass of water \((\rm M_{w})\) = 118 g
Specific heat capacity of water \((\rm C_{w})\) = \(4.184 \rm \;J/g^{\circ}\;\rm C\)
The initial temperature of water \((\rm T_{w})\) = \(1 ^{\circ}\;\rm C\)
Equilibrium temperature = \((\rm T_{e})\)
The equilibrium temperature can be shown as, heat loss by the gold = heat gained by the water:
\(\rm \rm M_{g}C_{g}(T_{g} - T_{e}) = M_{w}C_{w}(T_{e}-C_{w})\)
Substituting values in the equation:
\(\begin{aligned} 10 \times 0.129 (24 - \rm T_{e}) &= 118 \times 4.184 (\rm T_{e} - 1)\\\\\rm 1.29(24 - T_{e}) &= 493.712 (\rm T_{e} - 1)\\\\524.672 &= 495.002 \;\rm T_{e}\end{aligned}\)
Now divide both the sides by 495.002:
\(\begin{aligned} \rm T_{e} &= \dfrac{524.672 }{495.002}\\\\\rm T_{e} &= 1.06 \;^{\circ}\rm C\end{aligned}\)
Therefore, the final temperature of the system is 1.06 degrees celsius.
Learn more about equilibrium temperature here:
https://brainly.com/question/16207236
How many moles of nitrogen gas is 40.0 g?
Answers
Answer:
1.43 moles N₂
Explanation:
To determine the moles of nitrogen gas (N₂), you need to multiply the given value by the molar mass. The molar mass is a ratio comparing the mass (g) of nitrogen gas per 1 mole. It is important to arrange this ratio in a way that allows for the cancellation of units (grams should be in the denominator). The final answer should have 3 sig figs to match the given value.
Molar Mass (N₂): 2(14.007 g/mol)
Molar Mass (N₂): 28.014 g/mol
40.0 grams N₂ 1 mole
------------------------- x ------------------------ = 1.43 moles N₂
28.014 grams
What mass of NaCl is in 1.25L of 0.1035M solution?
Answers
Answer:
Explanation:
To determine the mass of NaCl in 1.25L of 0.1035M solution, we can use the formula:
mass = concentration x volume x molar mass
where concentration is in molarity (M), volume is in liters (L), and molar mass is in grams per mole (g/mol).
The molar mass of NaCl is 58.44 g/mol.
Plugging in the given values, we get:
mass = (0.1035 M) x (1.25 L) x (58.44 g/mol)
mass = 7.3188 g
So, there is approximately 7.3188 grams of NaCl in 1.25L of 0.1035M solution.
Following a chemical reaction that produced 5.06 grams of magnesium chloride, the lab report was prepared to
document the results. The expected result was estimated to be 8.85 grams. What are the percent yield and percent
error that are to be included in the lab report?
Percent Yield
Percent Error
Answers
Answer:
1. Percentage yield = 57.2%
2. Percentage error = 74.9%
Explanation:
From the question given above, the following data were obtained:
Actual yield = 5.06 g
Experimental yield = 8.85 g
Percentage yield =?
Percentage error =?
1. Determination of the percentage yield.
Actual yield = 5.06 g
Experimental yield = 8.85 g
Percentage yield =?
Percentage yield = Actual yield /Experimental yield × 100
Percentage yield = 5.06 / 8.85 × 100
Percentage yield = 57.2%
2. Determination of the Percentage error.
Actual yield = 5.06 g
Experimental yield = 8.85 g
Percentage error =?
Percentage error = |Experimental – Actual| / Actual yield × 100
Percentage error = |8.85 – 5.06| / 5.06 × 100
Percentage error = 3.79 / 5.06 × 100
Percentage error = 74.9%
Which solution contains exactly 0.25 mole of HCl?
Answers
The most typical solution is a 0.25 molar (M) HCl solution, which has exactly 0.25 moles of HCl in it. Per litre of this solution, 0.25 moles of HCl are present. Calculating the mass of HCl required to produce one litre of 0.25 M HCl solution is the first step in making this solution.
The molar mass of HCl (36.46 g/mol) must be multiplied by 0.25 moles to get this. As a result, we get 9.115 grammes of HCl in total mass. This quantity of HCl needs to be dissolved in one litre of water in order to make the solution.
This will result in a solution of 0.25 M HCl, or exactly 0.25 moles of HCl. It's critical to understand that a solution's concentration It's crucial to understand that a solution's concentration and its solute content are two different things.
For instance, an HCl solution at 0.5 M does not contain twice as much HCl as one at 0.25 M. It has twice as much solute in the same amount of solution, though. As a result, 0.5 moles rather than 0.25 moles of HCl are present in a 0.5 M solution.
Learn more about HCl at:
https://brainly.com/question/30233723
#SPJ1
when is an inferential control configuration needed? what do you think is its primary weakness? compare it to a simple feedback control configuration. which on is preferable?
Answers
By using inferential control, the difficult-to-measure controlled variables are approximated from certain simple process variables before being used in feedback control.
Which tool is employed to measure process variables?The two types of flow meters that are frequently used to measure the density rate of flow or velocity of a flowing fluid are the venturi meter and the orifice meter. They are also referred to as variable heading meters.
Which 5 factors are the primary process variables?An element of a physical or chemical amount that is typically monitored and managed throughout the operation of a sewage, wastewater, or industry treatment facility. Process variables like flow, level, tension, temp, turbidity, chlorine, & oxygen concentrations are frequently used.
To know more about measure controlled variables visit:
https://brainly.com/question/438542
#SPJ1
What is happening to the electrons between several atoms of Copper?
A.nothing happens to the electrons
B.the electrons become delocalized and form a "sea"
C.they are shared between two atoms
D.the electrons are transferred from one atom to another
Answers
Answer:
\(\color{Blue}\huge\boxed{Answer} \)
B.the electrons become delocalized and form a "sea"
does the molecule which have C2 axis perpendicular to the Cn axis have mirror plane perpendicular to the Cn axis ?
Answers
No, a molecule with a \(C_2\)axis perpendicular to the \(C_n\)axis does not necessarily have a mirror plane perpendicular to the \(C_n\)axis.
The presence of a \(C_2\)axis perpendicular to the \(C_n\)axis implies that the molecule possesses rotational symmetry around the \(C_n\)axis. However, the presence of a mirror plane is determined by the presence of an additional symmetry element in the molecule.
A mirror plane is a symmetry element that divides the molecule into two halves, with one half being the mirror image of the other. In order for a mirror plane to be present perpendicular to the \(C_n\)axis, there needs to be an additional symmetry element that produces the reflection symmetry.
While a molecule with a \(C_2\) axis perpendicular to the \(C_n\)axis has rotational symmetry, it does not necessarily possess reflection symmetry. For example, consider a molecule with a \(C_2\)axis perpendicular to a \(C_3\)axis.
The rotational symmetry is evident, as the molecule can be rotated by 120 degrees around the \(C_3\) axis and still appear the same. However, this molecule does not possess a mirror plane perpendicular to the \(C_3\)axis.
The presence of a mirror plane perpendicular to the \(C_n\)axis depends on the specific molecular geometry and arrangement of atoms. It is possible for a molecule to possess both rotational symmetry and a mirror plane perpendicular to the \(C_n\)axis, but it is not a general rule.
For more such questions on perpendicular visit:
https://brainly.com/question/23828050]
#SPJ8
CHEMISTRY HELP!
using only the periodic table, determine the charge on the ion that is formed by arsenic.
The ion charge is:
a. -3
b. -2
c. -1
d. 0
e. +1
f. +2
g. +3
also what is it for elements lithium and strontium?
Answers
Answer:
A
Explanation:
Arsenic is in the same group as Nitrogen - group 5. They all have 5 valence electrons in their outermost shell. To achieve its most stable state - 8 valence electrons (octet rule - elements are most stable when the entire shell is filled) - arsenic needs to gain 3 electrons. Since electrons have a negative charge, the charge of an As ion would be -3.
Try observing the periodic table and how many valence electrons that each element has. From there, you can determine the charges of the elements lithium and strontium. You can guess, I'll help you with those once you attempt to find the charge of those ions.
How many grams of NaCl

Answers
You would recover 36.525g of NaCl after evaporating all of the water.
How to find the how many grams of NaCl that would be recover when all water is evaporated off of this solution?To find the grams of NaCl that would be recovered after evaporating all the water, we can use the following formula:
mass = moles * molar mass
Where:
Moles = Molarity * Volume
Molarity = 0.250 M
Volume = 2500.0 mL = 2.5 L
Molar mass of NaCl = 58.44 g/mol
mass = 0.250 M * 2.5 L * 58.44 g/mol
mass = 36.525 g
Learn about evaporation here https://brainly.com/question/2013258
#SPJ1
HỌ5,42
Homework Answered Due Today, 11:59 PM
.
A 5.60E1 g sample of water at 9.910E1 °C is placed in a constant pressure calorimeter. Then, 2.40E1 g of zinc metal at 2.10E1 °C is
added to the water and the temperature drops to 9.70E1 °C. What is the specific heat capacity of the zinc metal measured in this
experiment?
Answers
The specific heat capacity of the zinc metal, given that 2.40×10¹ g of zinc metal at 2.10×10¹ °C is added to the water is 0.27 J/gºC
How do i determine the specific heat capacity of the zinc?First, we shall obtain the heat absorbed by the water when the zinc metal was added. This is shown below:
Mass of water (M) = 5.60×10¹ gInitial temperature (T₁) = 9.910×10¹ °CFinal temperature (T₂) = 9.70×10¹ °CChange in temperature (ΔT) = 9.70×10¹ - 9.910×10¹ = -2.1 °CSpecific heat capacity of water (C) = 4.184 J/gºC Heat absorbed by water (Q) =?Q = MCΔT
= 5.60×10¹ × 4.184 × -2.1
= -492.0384 J
Now, we shall determine the specific heat capacity of the zinc metal. Details below:
Heat absorbed by water (Q) = -492.0384 JHeat released by metal (Q) = 492.0384 JMass of zinc metal (M) = 2.40×10¹ gInitial temperature (T₁) = 2.10×10¹ °CFinal temperature (T₂) = 9.70×10¹ °CChange in temperature (ΔT) = 9.70×10¹ - 2.10×10¹ = 76 °CSpecific heat capacity (C) = ?Q = MCΔT
492.0384 = 2.40×10¹ × C × 76
492.0384 = 1824 × C
Divide both sides by 1824
C = 492.0384 / 1824
= 0.27 J/gºC
Learn more about specific heat capacity:
https://brainly.com/question/19104255
#SPJ1
The following reaction shows sodium carbonate reacting with calcium hydroxide.
Na2CO3 + Ca(OH)2 -> 2NaOH + CaCO3
How many grams of NaOH are produced from 20.0 grams of Na2CO3?
(Molar mass of Na = 22.989 g/mol, C = 12.01 g/mol, O = 15.999 g/mol, Ca = 40.078 g/mol, H = 1.008
g/mol) (5 points)
Answers
Answer:
Its 15.1
Explanation:
Answer:
15.1
Explanation:
Balance Equation: Na2CO3 + Ca(OH)2 = 2NaOH + CaCO3
Stoichiometry:
20gNa2CO3/1 x 1mNa2CO3/105.985gNa2CO3 x 2mNAOH/1mNA2CO3 x 39.996gNaOH/1mNaOH = 15.1 grams of NaOH
-Convert 6.02 x 1020 formula units of MgCl₂ to mol of MgCl₂:
Answers
6.02 x \(10^{20\) formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
To convert formula units of MgCl₂ to moles of MgCl₂, we need to use Avogadro's number, which relates the number of formula units to the number of moles.
Avogadro's number (NA) is approximately 6.022 x 10^23 formula units per mole.
Given that we have 6.02 x 10^20 formula units of MgCl₂, we can set up a conversion factor to convert to moles:
(6.02 x 10^20 formula units MgCl₂) * (1 mol MgCl₂ / (6.022 x 10^23 formula units MgCl₂))
The formula units of MgCl₂ cancel out, and we are left with moles of MgCl₂:
(6.02 x 10^20) * (1 mol / 6.022 x 10^23) = 0.1 mol
Therefore, 6.02 x 10^20 formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
It's important to note that this conversion assumes that each formula unit of MgCl₂ represents one mole of MgCl₂. This is based on the stoichiometry of the compound, where there is one mole of MgCl₂ for every one formula unit.
Additionally, this conversion is valid for any substance, not just MgCl₂, as long as you know the value of Avogadro's number and the number of formula units or particles you have.
For more such questions on MgCl₂ visit:
https://brainly.com/question/26311875
#SPJ8
1. If 4.0 L of gas in the lungs have a pressure of 1.0 atm and are kept at body temperature (37 degrees Celsius), how many
moles are present in the lungs? Show your work.
Answers
There are approximately 0.16 moles of gas present in the lungs.
Steps
To solve this problem, we can use the ideal gas law, which relates the pressure (P), volume (V), number of moles (n), and temperature (T) of a gas:
PV = nRT
where R is the gas constant.
First, we need to convert the temperature to Kelvin:
T = 37°C + 273.15 = 310.15 K
Next, we can plug in the given values:
P = 1.0 atm
V = 4.0 L
T = 310.15 K
We also need to find the value of R, which depends on the units we are using for pressure, volume, and temperature. In this case, we are using atmospheres, liters, and Kelvin, so we can use the value:
R = 0.08206 L·atm/K·mol
Now we can rearrange the ideal gas law to solve for the number of moles:
n = PV/RT
n = (1.0 atm)(4.0 L)/(0.08206 L·atm/K·mol)(310.15 K)
n = 0.1638 mol
Therefore, there are approximately 0.16 moles of gas present in the lungs.
learn more about pressure here
https://brainly.com/question/28012687
#SPJ1
Which model is useful in showing the reactivity of potassium?
Answers
Answer:we need the pic bro
Explanation:
which is a positive aspect of fossil fuels? help now
Natural Resources Unit Test
4 of 164 of 16 Items
Question
Which is a positive aspect of fossil fuels?(1 point)
Responses
They can be recycled and reused.
They can be recycled and reused.
They provide energy for cars, trains, and heat.
They provide energy for cars, trains, and heat.
They are ecologically friendly.
They are ecologically friendly.
They do not need processing to produce energy.
They do not need processing to produce energy.
Answers
Positive aspect of fossil fuels is They provide energy for cars, trains and heat.
1) They can be recycled and reused . this point is wrong because fossil fuels are non renewable resources and are in limits in supply . so, fossil fuels cannot be recycled.
2) They provide energy for cars, trains, and heat. This is statement is true.
3) They are ecologically friendly. this statement is wrong. The fossil fuels are not eco-friendly . they produce the pollution to the environment and severely damage our environment.
4) They do not need processing to produce energy. they need geological process of millions years.
Thus, Positive aspect of fossil fuels is They provide energy for cars, trains and heat.
To learn more about Fossil fuels here
https://brainly.com/question/2029072
#SPJ1
(giving brainlist! What should a simplified model of a large molecule like glucose show?
Answers
Glucose is a carbohydrate and monosaccharide sugar molecule that is the energy source for organisms. The simplified model of a glucose molecule will show six carbon atoms, six oxygen atoms, and twelve hydrogen atoms.
What is glucose?Glucose is one of the monosaccharide sugar that is the most abundant energy source with a molecular formula of C₆H₁₂O₆. It consists of carbon, hydrogen, and oxygen atoms with a molecular mass of 180.156 g/mol.
It is a sugar-containing aldehydic group with a six-carbon making it aldohexoses. It is found in honey and fruits that are the source of the energy and regulates metabolic and cellular reactions.
Therefore, the molecular structure of glucose contains six carbon, twelve hydrogens, and six oxygen atoms.
Learn more about glucose here:
https://brainly.com/question/2396657
#SPJ2
At what state is bromine at 100 degree
Answers
Answer:
a liquid
Explanation:
Name the following ketone:
o
CH3CCH3

Answers
Answer: the answer is A
Explanation:
just finished this on acellus
Acetone is the name of the following compound and can also be referred to as 2-propanone.
What is a ketone?ketone, any of a class of organic compounds characterized by the presence of a carbonyl group in which the carbon atom is covalently bonded to an oxygen atom.
Acetone, an organic compound, is chemically described as \(CH_3COCH_3\), and can also be referred to as 2-propanone.
Acetone is a hydrocarbon derivative, more specifically a ketone in its simplest form because of its particular functional group.
Hence, acetone is the name of the following compound and can also be referred to as 2-propanone.
Learn more about the ketone here:
https://brainly.com/question/4439718
#SPJ2
A compound is found to contain 9.227 % boron and 90.77 % chlorine by mass. What is the empirical formula for this compound?
Answers
Assuming a 100 g sample of the compound, we can convert the mass percentages to masses in grams:
- 9.227 g B
- 90.77 g Cl
Next, we need to convert these masses to moles using the atomic masses of the elements:
- B: 10.81 g/mol
- Cl: 35.45 g/mol
- 9.227 g B ÷ 10.81 g/mol = 0.853 mol B
- 90.77 g Cl ÷ 35.45 g/mol = 2.562 mol Cl
Now we need to divide both mole values by the smaller of the two, which is 0.853 mol:
- 0.853 mol B ÷ 0.853 mol = 1.000 mol B
- 2.562 mol Cl ÷ 0.853 mol = 3.000 mol Cl
This gives us a B:Cl ratio of 1:3. The empirical formula for the compound is therefore BCl3.
Answer:
Empirical formula of a compound means that it provides simplest ratio of whole number.
Explanation:
Mass of boron and chlorine is 9.224% and 90.74%
A student conducted three trails to determine the concentration of an unknown concentration of HCI. In the first trail the calculated concentration was 0.104 M, the second was 0.113 M and the third trail was 0.108 M. What is the percent difference between the first two trials and based on the lab procedures procedures guidelines what would the average molarity be?
Answers
The percent difference between the first two trials is 8.3% and final outcome would be an average molarity of 0.108 M.
How to calculate percent difference and average molarity?To calculate the percent difference between the first two trials, use the formula:
% Difference = |(Value 1 - Value 2) / ((Value 1 + Value 2) / 2)| x 100%
% Difference = |(0.104 M - 0.113 M) / ((0.104 M + 0.113 M) / 2)| x 100%
% Difference = |-0.009 M / 0.1085 M| x 100%
% Difference = 8.3%
The percent difference between the first two trials is 8.3%.
To find the average molarity, add the three calculated concentrations together and divide by the number of trials:
Average Molarity = (0.104 M + 0.113 M + 0.108 M) / 3
Average Molarity = 0.108 M
Based on the lab procedures guidelines, the average molarity would be the most accurate representation of the unknown concentration of HCI. Therefore, the average molarity of 0.108 M would be the final result.
Find out more on percent difference here: https://brainly.com/question/10791047
#SPJ1
28.25 What two aldoses yield D-xylose on Wohl degradation?
Answers
56.5
Explanation:
the degradation would be 28.25 times 2
Using the guideline for oxidation numbers, write the reduction half-reactions for the following:
• O
• P
• Cu
Answers
The reduction half-reactions for O, P, and Cu:
• O: O2 + 4 e- → 2 O2-
• P: HPO42- + 2 H+ + 2 e- → H3PO4
• Cu: Cu2+ + 2 e- → Cu+
To write the reduction half-reactions for O, P, and Cu, we need to determine the oxidation numbers for each element. The guidelines for assigning oxidation numbers are:
The oxidation number of an atom in its elemental form is 0.The oxidation number of a monatomic ion is equal to its charge.The sum of the oxidation numbers of all atoms in a neutral molecule must be 0.The sum of the oxidation numbers of all atoms in a polyatomic ion must be equal to the charge of the ion.Using these guidelines, we can determine the oxidation numbers for O, P, and Cu:
O: Oxygen is a diatomic molecule, so its oxidation number is 0 in O2.P: The most common oxidation state for phosphorus is +5 in its compounds, but it can also have oxidation states ranging from -3 to +5.Cu: The most common oxidation state for copper is +2, but it can also have oxidation states ranging from +1 to +4.To know more about the Reduction half-reaction, here
https://brainly.com/question/18403544
#SPJ4