What will be the freezing point of a solution that contains 60.5g of glucose (C6H12O6) in 555g of water
Answers
The freezing point of a solution that contains 60.5g of glucose (C6H12O6) in 555g of water will be -1.25°C.
The freezing point of a solution that contains 60.5g of glucose (C6H12O6) in 555g of water can be found using the formula
ΔTf = Kf x molality,
where ΔTf is the freezing point depression, Kf is the freezing point depression constant and molality is the concentration of the solution in moles of solute per kilogram of solvent.
The molar mass of glucose is 180.16 g/mol, so the number of moles of glucose in the solution is:
60.5 g / 180.16 g/mol = 0.336 mol
The mass of water in the solution is:
555 g - 60.5 g = 494.5 g
The molality of the solution is therefore:
0.336 mol / 0.4945 kg = 0.679 m
The freezing point depression constant for water is 1.86 °C/m, so the freezing point depression of the solution is:
ΔTf = 1.86 °C/m x 0.679 m = 1.26494 °C
Rounding off to the nearest 100th, the freezing point of the solution is
0.01 - 1.26 = -1.25°C.
The freezing point of a solution that contains 60.5g of glucose (C6H12O6) in 555g of water will be -1.25°C.
To know more about freezing point visit:
https://brainly.com/question/31357864
#SPJ11
Related Questions
Sulfuric acid is very corrosive and used in cleaning agents such as drain cleaners. How many moles are in 2.11 x 10^22 molecules of H₂SO4?
Answers
The number of moles in 2.11 x \(10^{22\) molecules of \(H_2SO_4\) would be 0.035 mol.
Number of moles in moleculesAccording to the established standard by Avogadro, there is 1 mole in 6.022 x \(10^{23\) molecules of substances. This is irrespective of the substance.
6.022 x \(10^{23\) molecules = 1 mole
Now, the equivalent number of moles in 2.11 x \(10^{22\) molecules of \(H_2SO_4\) would be:
2.11 x \(10^{22\)/6.022 x \(10^{23\) = 2.11/60.22 = 0.035 mol
In other words, the number of moles in 2.11 x \(10^{22\) molecules of \(H_2SO_4\) is 0.035 mol.
More on moles in molecules of substances can be found here: https://brainly.com/question/14585733
#SPJ1
Monica put four items on the desk that you need to measure the mass of. The items are listed below. Which of the following would need to be put in a container in order to measure the mass on a scale?
I. Flour
II. Sugar cubes
III. Milk
IV. An unbroken egg
A. I and III
B. I and IV
C. II and III
D. III and IV
Answers
Answer:
it D because it make more sense
what determines the role of an organism in an ecosystem?

Answers
Answer:
An organism's role within an ecosystem depends on how it obtains its food. Plants and animals obtain their food in very different ways, so they have very different roles in an ecosystem. The way in which an organism obtains food also affects its interactions with other organisms in the ecosystem.
Explanation:
. Why is it important to know the conditions under which an aqueous redox reaction takes place in order to balance the ionic equation for the reaction
Answers
Answer:
Just like with any other reaction, balancing the equation for the reaction is important so that people can know all the inputs and outputs, and know how much of each input (reactant) is needed to produce a certain amount of product.
number the steps for balancing equations:
Use coefficients to increase the atoms on each side.
Check to make sure you have the same number of each type of atom on each side.
Count the atoms on each side.
ldentify the atoms on each side.
Answers
Answer:
identify the atoms on each side.
Count the atoms on each side.
Use coefficients to increase the atoms on each side.
Check to make sure you have the same number of each type of atom on each side.
Explanation:
The concept behind balancing chemical equations is to ensure that they comply with the law of conservation of matter. This helps to make chemical equations quantitatively meaningful.
First, identify the atoms on each side of the expression. Then count these atoms. Assign appropriate numeric coefficient to the species. Then check to make sure there are equal number of each type of atoms on each side.The subscript of the formula must not be changed in an attempt to balance a chemical equation.
Surface tension _____. A. Causes beads of water to spread out B. May be increased by detergents C. Is an inward force that tends to minimize the surface area of a liquid D. Is decreased by hydrogen bonding
Answers
Answer:
C. Is an inward force that tends to minimize the surface area of a liquid
Explanation:
Surface tension is a skin-like proper of water that is explained by its property or ability to form hydrogen bonds. The water molecule has the ability to bind the molecules from all sides that surrounds it. The force of action on each of the molecules is balanced. The surface tension is a more inward phenomenon and it holds the droplet in a spherical shape.if an ion has 26 protons and 24 electrons what is the charge on the ion?
Answers
Answer:
+2
Explanation:
Protons are positive and electrons are negative, this ion has more positive charges than negative charges therefore the overall charge of the ion is +2.
+26 + -24 = +2
If an ion has 26 protons and 24 electrons, the charge of the ion will be +2.
HOW TO DETERMINE CHARGE OF AN ION:
The charge of an ion can be determined by subtracting the number of electrons from the number of protons in the atom. Charge = no. of protons - no. of electronsAccording to this question, an ion has 26 protons and 24 electrons. This means that the charge of the ion can be calculated as follows:Charge of ion = 26 protons - 24 electrons = +2. Therefore, if an ion has 26 protons and 24 electrons, the charge of the ion will be +2.Learn more about how to calculate charge of an ion at: https://brainly.com/question/14471616?referrer=searchResults
Which of these industrial processes typically involves electrolysis?
purifying water for drinking purposes
purifying copper to produce copper wiring for homes
neutralizing an acidic solution by adding a base
neutralizing a basic solution by adding an acid
Answers
Answer:
purifying copper to produce copper wiring for homes
Explanation:
Answer:
b.purifying copper to produce copper wiring for homes
Explanation:
The mass of neutrons and protons is much greater than the mass of electrons; therefore the mass of an element is dominated by the mass of the nucleus. Predict which, if any, types of snacks will dominate the mass of your imaginary element.
Answers
Since the nucleus houses the neutron and the proton, it then follows that the nucleus is the point where most of the mass of the atom is concentrated.
What is the nucleus?The nucleus is composed of protons and neutrons. We know that the mass of the proton is 1640 times the mass of the electron. The neutron only contributes to the mass of the atom.
Since the nucleus houses the neutron and the proton, it then follows that the nucleus is the point where most of the mass of the atom is concentrated.
The electron has a negligible mass therefore, the electron does not contribute to the mass of the atom.
Learn more about nucleus:https://brainly.com/question/23366064
#SPJ1
The illustration to the left represents a mixture of hydrogen ( light blue ) and oxygen ( red ) reacting to form a product.
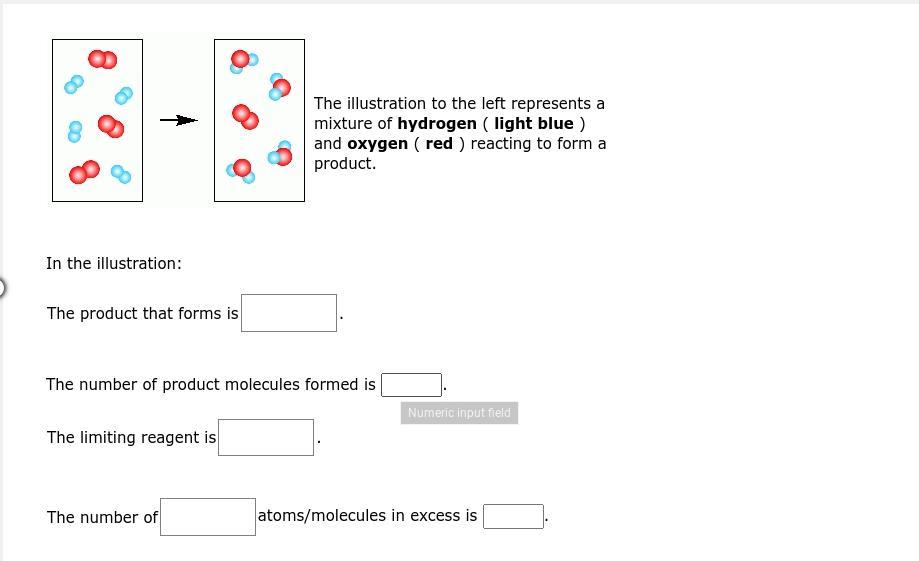
Answers
The product formed is H\(_2\)( g) + O\(_2\)( g) → 2 H\(_2\) O ( l). There are two moles of water that is formed when hydrogen and oxygen react.
What is hydrogen?The simplest chemical element is hydrogen (H), a colorless, odorless, tasteless, and combustible gaseous substance. The nucleus of the hydrogen atom is composed of a proton with one unit of positively electrical current and an electron with one unit of negatively electrical charge.
Under normal circumstances, hydrogen gas is a loose collection of hydrogen molecules, of which each is a diatomic molecule, or H2, made up of two atoms. The product formed is H\(_2\)( g) + O\(_2\)( g) → 2 H\(_2\) O ( l). There are two moles of water that is formed when hydrogen and oxygen react.
Therefore, the product formed is H\(_2\)( g) + O\(_2\)( g) → 2 H\(_2\) O ( l). There are two moles of water that is formed when hydrogen and oxygen react.
To learn more about hydrogen, here:
https://brainly.com/question/19678094
#SPJ1
Scientists improved Mendeleev’s early periodic table by ___.
including physical properties of elements such as melting point and density
including chemical properties of elements such as the ability to burn or to tarnish
using atomic number instead of atomic mass to organize the elements
using numbers of neutrons and protons to organize the elements by their properties
Answers
Scientists improved Mendeleev’s early periodic table by using atomic number instead of atomic mass to organize the elements. Option C
What is the periodic table?We know that the periodic table is the arrangement of the elements by the use of a definite sequence. The order of the arrangement of the elements would be determined in a given manner.
In the Mendeleev’s periodic table, the arrangement of the elements was on the basis of the masses of the elements. This method did not produce a consistent pattern for all the elements.
Learn more about the periodic table:https://brainly.com/question/11155928
#SPJ1
Answer:using atomic number instead of atomic mass to organize the elements
Question 1 of 10
Which two factors affect the amount of thermal energy an object has?
A. The directions in which its particles are moving
B. The mass of the object
O C. The amount of motion its particles have
D. The amount of space between its particles
Answers
Answer:
b, and d
Explanation:
because it makes the most sense I might be wrong
What do nuclear reactions change in the atom? covalent bonds ionic bonds electrons neutrons protons
Answers
A nuclear reaction changes the number of protons and or neutrons in an atom.
What do nuclear reactions change in the atom?A nuclear reaction is a type of reaction that results in the change of the nucleus of an atom. We know that a nucleus of an atom is made up of protons and neutrons.
So we can conclude that a nuclear reaction changes the number of protons and or neutrons in an atom.
Learn more about reaction here: https://brainly.com/question/26018275
#SPJ4
Answer: Nuclear reactions happen inside the nucleus,so it changes the protons and neutrons
Explanation:
Explain why C6H6 is a Lewis base, but not a Bronsted Lowry or Arrhenius base.
Answers
Answer:
C6H6, also known as benzene, is a Lewis base because it can donate a pair of electrons to form a coordinate covalent bond with a Lewis acid. A Lewis base is defined as any substance that can donate a pair of electrons to form a coordinate covalent bond.
However, benzene is not a Bronsted-Lowry base because it does not have a hydrogen ion (H+) to donate. A Bronsted-Lowry base is defined as any substance that can donate a hydrogen ion (H+).
Benzene is also not an Arrhenius base because it does not produce hydroxide ions (OH-) when dissolved in water. An Arrhenius base is defined as any substance that produces hydroxide ions (OH-) when dissolved in water.
Explanation:
There are different definitions of what a base is. Three common definitions are the Lewis, Bronsted-Lowry, and Arrhenius definitions.
According to the Lewis definition, a base is any substance that can donate a pair of electrons to form a bond. Benzene (C6H6) can do this, so it is considered a Lewis base.
The Bronsted-Lowry definition says that a base is any substance that can donate a hydrogen ion (H+). Benzene does not have a hydrogen ion to donate, so it is not considered a Bronsted-Lowry base.
The Arrhenius definition says that a base is any substance that produces hydroxide ions (OH-) when dissolved in water. Benzene does not produce hydroxide ions when dissolved in water, so it is not considered an Arrhenius base.
Sequence Examine the periodic table.
Which four pairs of elements would be
reversed in order if the elements were
listed by increasing atomic mass instead of
increasing atomic number?
Answers
Answer:
Notice that tellurium is listed before iodine even though its atomic mass is higher. Mendeleev reversed the order because he knew that the properties of iodine were much more similar to those of fluorine (F), chlorine (Cl), and bromine (Br) than they were to oxygen (O), sulfur (S), and selenium (Se).
The tellurium is listed before iodine even though its atomic mass is higher. Mendeleev reversed the order because he knew that the properties of iodine were much more similar to those of fluorine (F), chlorine (Cl), and bromine (Br) than they were to oxygen (O), sulfur (S), and selenium (Se).
What things are in the periodic table?The periodic table is a tabular Form of the chemical elements classified by atomic number, from the element with the least atomic number, hydrogen, to the element with the most atomic number.
The atomic number is the number of protons in the nucleus of an atom.
Thus, The tellurium is listed before iodine even though its atomic mass is higher. Mendeleev reversed the order because of its properties.
To learn more about periodic table click here:
https://brainly.com/question/11155928
#SPJ2
Classify Mg(OH)2 as a
strong base or a weak base?
Strong Base
Weak Base

Answers
Answer:
Explanation:
Answer
It's a very strong base. The closer you are to column 1 you are, the stronger the base.
Explain the mechanism of a Horner-Wadsworth-Emmons reaction between diethyl benzylphosphonate and 3,4-methylenedioxybenzaldehyde in the presence of aqueous NaOH forming 3,4-methylenedioxystilbene as the product.
Answers
Horner-Wadsworth-Emmons (HWE) reaction is an important synthetic reaction in organic chemistry. It is widely used for synthesizing various compounds. The reaction is between an aldehyde or ketone and a phosphonate or phosphonate ester in the presence of a strong base.
The Horner-Wadsworth-Emmons reaction is one of the most convenient and well-known methods of constructing carbon-carbon double bonds. The reaction proceeds via the formation of an ylide intermediate. The HWE reaction is particularly useful for the synthesis of compounds with a Z-configuration.
The mechanism for the reaction of diethyl benzylphosphonate and 3,4-methylenedioxybenzaldehyde in the presence of aqueous NaOH, forming 3,4-methylenedioxystilbene as the product, can be explained in the following steps:
Step 1: Formation of the ylide intermediate
The reaction starts with the formation of an ylide intermediate. This is achieved by the reaction of diethyl benzylphosphonate and 3,4-methylenedioxybenzaldehyde in the presence of a strong base like NaOH or KOH. In this reaction, a deprotonated species called an ylide intermediate is generated.
Step 2: Addition of the ylide intermediate to the aldehyde
The ylide intermediate then attacks the aldehyde, leading to the formation of a betaine intermediate.
Step 3: Formation of the phosphonate ester
The betaine intermediate undergoes elimination to form the final product, 3,4-methylenedioxystilbene, and the by-product phosphonate ester.
The mechanism of the Horner-Wadsworth-Emmons (HWE) reaction between diethyl benzylphosphonate and 3,4-methylenedioxybenzaldehyde in the presence of aqueous NaOH, forming 3,4-methylenedioxystilbene as the product, is complete. This reaction is significant in organic chemistry and finds applications in the pharmaceutical industry.
Learn more about reaction
https://brainly.com/question/30464598
#SPJ11
A sample of ammonia gas is stored in a 227 ml flask at 600 mm of Hg and 10.0°C. The gas is allowed to expand into another flask of volume 1.78L at 27°C. What is the pressure of ammonia in the second flask ?
Answers
Answer:
P1V1/T1 = P2V2 / T2.
- combined gas
equation
(227×600) / 283. = (1780× P2 )/300
P2 = (300 ×227×600)/283 ×1780
P2 = 81.11
Hope this helps!
Don't forget to mark me as Brainliest.
Experimental
is a factor that can arise from incorrect use
of measuring tools or malfunctioning equipment..
Answers
Answer:
Error.
Explanation:
An experiment can be defined as an investigation which typically involves the process of manipulating an independent variable (the cause) in order to be able to determine or measure the dependent variable (the effect).
This ultimately implies that, an experiment can be used by scientists to show or demonstrate how a condition causes or gives rise to another i.e cause and effect, influence, behavior, etc in a sample.
Cause and effect can be defined as the relationship between two things or events in which an occurrence one (cause) leads to the occurrence of another (effect).
Experimental error is a factor that can arise from incorrect use of measuring tools or malfunctioning equipment such as thermometer, barometer, multimeter, voltmeter, ammeter, vernier caliper, etc. This error usually causes test results to be inaccurate, incorrect and as such leading to wrong experimental conclusions.
Also, one common example of an experimental error is the error due to parallax.
please help!!!!!!!!!

Answers
Answer:
H2O (2x1.008 + 1x15.999) = 18.015 g/mol
CO2 (1x12.011 + 2x15.999) = 44.01 g/mol
BF3 (1x10.81 + 3x18.998) = 67.81 g/mol
K2O (2x39.098 + 1x15.999) = 94.20 g/mol
BaCO3 (1x137.33 + 1x12.011 + 3x15.999) = 197.34 g/mol
New crust is found at the center of the _____, proving the movement of the seafloor and the surface of the Earth.
A. ocean
B. Himalayan Mountains
C. Earth
D. mid-ocean ridge
Answers
Answer: The newest, thinnest crust on Earth is located near the center of mid-ocean ridge—the actual site of seafloor spreading
Answer:
D. mid-ocean ridge
Explanation:
Brainliest if correct!!!!!
Determine which of the following statements is true.
A. A catalyst causes equilibrium to be reached faster without changing the position of the equilibrium.
B. A catalyst causes the reaction to move more slowly so that the equilibrium position can be precisely adjusted.
C. A catalyst raises the activation energy of a reaction.
D. A catalyst speeds up the rate of the forward reaction which moves the equilibrium towards the products.
Answers
Answer:
A
Explanation:
A catalyst speeds up a reaction by lowering the required activation energy, so it's not B. Like previously stated, the catalyst lowers the activation energy required, so it's not C either. This leaves us with A. Just to double check, does a catalyst speeds up a reaction? Yes! So like it says in A, a catalyst causes the equilibrium to be reached faster. Have a nice day! :)
Catalysts speed up a chemical reaction by causing equilibrium to reach quickly while maintaining the equilibrium's position. Thus, option A is true.
What are catalysts?
A catalyst increases the reaction rate by decreasing the activation energy of the reaction. As the minimum energy required by the reactants for the reaction is decreased the rate of the formation of products increases.
During the reaction, the catalyst remains unchanged and does not undergo any chemical change. It can increase the reaction rate of both irreversible and reversible reactions.
In an equilibrium reaction, the catalyst can increase the rate of both forward and reverse reaction by reaching the reaction equilibrium at a faster rate and can move the reaction toward the products as well as a reactant.
Therefore, the catalyst causes equilibrium to be reached faster.
Learn more about catalysts here:
https://brainly.com/question/22363846
#SPJ5
Correct ANSWER GETS BRAINLIST

Answers
Answer:
The answer is B lose photosynthetic ability.
Explanation:
The acidity of acid rain can directly affect the plant leaves necessary for photosynthesis, and it makes the soils unable to sustain plant life as well. Instead of letting plants grow quicker it actually slows down plant growth.
Which set of coefficients will balance this chemical equation?
__C2H4(g) + __O2(g) → 2CO2(g) + 2H2O(g)
Answers
Answer:
Explanation:
USE UR PERDICIRTON TABLE
The tails of animals can serve many important functions. They are mainly used in balance and locomotion. Many lizards have a fragile, detachable tail that will come off when they are attacked by predators, allowing them to escape.
Why is the presence of a brightly colored, detachable tail an advantage for some lizards?

Answers
Answer:
The presence of a brightly colored, detachable tail can be advantageous for some lizards because it serves as a distraction or decoy for predators. When a predator attacks, the lizard can quickly detach its tail, which wriggles and continues to move for a short period of time. This sudden movement can divert the predator's attention away from the lizard's body, allowing the lizard to escape.
The bright coloration of the tail can also help to draw the predator's attention towards it, instead of the lizard's body. Bright colors are often used in the animal kingdom as warning signals, indicating that an animal is toxic or dangerous. While the lizard may not necessarily be toxic or dangerous, the bright colors of its tail can create a similar effect, deterring predators from attacking in the first place.
Additionally, the detachable tail can serve as a form of self-defense, allowing the lizard to escape from a predator's grasp. By detaching its tail, the lizard can leave the predator with a distraction while it makes its escape. Over time, the lizard can regrow its tail, allowing it to continue to use this defense mechanism in the future.
the radius of a single atom of a generic element x is 123 picometers (pm) and a crystal of x has a unit cell that is body-centered cubic. calculate the volume of the unit cell.
Answers
The radius of a single atom of a generic element x is 123 picometers (pm) and a crystal of x has a unit cell that is body-centered cubic. So, the volume of the unit cell is 11.5482 x 10⁻²⁴ cm³.
Given,
The radius of a single atom of a generic element x is 123 picometers (pm) and a crystal of x has a unit cell that is body-centered cubic.
Body-Centered Cubic (BCC):
In a Body-Centered Cubic unit cell, each corner of the cube has a corner atom, and there is an additional atom in the center of the cube. The atom that is centered on the unit cell is surrounded by eight neighboring atoms, each of which is located at a distance of
4R/√3,
where R is the radius of the atom.
The volume of the unit cell = (4 * radius of the atom)^3/3
For BCC, volume of the unit cell is
(4 * radius of the atom)^3/3
= (4 * 123 pm)^3/3
= 11.5482 x 10⁻²⁴ cm³
The volume of the unit cell is 11.5482 x 10⁻²⁴ cm³.
For more such questions on volume , Visit:
https://brainly.com/question/29493123
#SPJ11
slove this...........................

Answers
Explanation:
................easy ngl

Answer:
for protons going down it is:
(already given)
6
6
8
8
17
17
for neutrons going down it is:
6
7
8
(already given)
10
18
20
for electrons going down it is:
6
6
8
8
(already given)
17
Explanation:
bascially the number of protons is the bottom number to the left and the number of electrons is the same as the number of protons and the number of neutrons is basically the mass number (the top right one) minus the proton number
hope this helps:)
Mg + 2 HCl ➞ MgCl2 + H2
How many grams of MgCl2 are produced by 2.55 mol Mg?
Answers
2.55 mol Mg yields 242.89 grams of MgCl2.
What volume of MgCl2 will be generated?Because MgCl2 and Mg have a 1:1 molar ratio, every time 1 mole of Mg interacts, 1 mole of MgCl2 is also created. As a result, the amount of Mg that reacts can be used to compute the number of moles of MgCl2 that are created.
HCl + 2 Mg + MgCl2 + H2
The amount of moles of MgCl2 that are created when 2.55 mol of Mg react can be estimated as follows: 2.55 mol Mg (1 mol MgCl2/1 mol Mg) = 2.55 mol MgCl2.
Now, we need to multiply the number of moles by the molar mass of MgCl2, which is 95.21 g/mol, in order to convert moles of MgCl2 to grams.
242.89 g MgCl2 from 2.55 mol MgCl2 and 95.21 g/mol
As a result, 2.55 mol of magnesium will result in 242.89 g of MgCl2.
To know more about mol visit:-
https://brainly.com/question/1504637
#SPJ1
what is allotropy in chemistry
Answers
The Dutch scientist Johannes van der Waals developed a useful equation to predict the behavior of real gases. In the van der Waals equation, what are the constants a and b, respectively?
a. a is a measure of how strongly the gas molecules attract one another, and b is a measure of the finite volume occupied by the molecules.
b. a is a measure of the random motion of gas molecules, and b is a measure of the volume of the container.
c. a is a measure of the finite volume occupied by the molecules, and b is a measure of how strongly the gas molecules attract one another.
d. a is a measure of the molecular mass of the gas molecules, and b is a measure of the finite volume occupied by the molecules.
Answers
Answer:
Explanation:
The higher the value of a, the greater the attraction between molecules and the more easily the gas will compress.
The b term represents the excluded volume of the gas or the volume occupied by the gas particles.