What Volumes Should You Expect For Fraction 1 2 And 3 For Fractional Distillation?
Answers
Fractional distillation is a technique used to separate mixtures of liquids with different boiling points.
The mixture is heated in a flask and the vapors are collected and condensed into separate fractions. Each fraction corresponds to a different boiling point and therefore a different component of the mixture.
The volumes of each fraction will depend on the composition of the mixture and the efficiency of the distillation process. However, you can expect the volume of fraction 1, which corresponds to the component with the lowest boiling point, to be the largest. This is because the component with the lowest boiling point will vaporize first and therefore be collected in the greatest amount.
The volumes of fractions 2 and 3 will be smaller, as they correspond to components with higher boiling points that will vaporize later in the distillation process. Overall, the volumes of each fraction will depend on the composition of the mixture and the efficiency of the distillation process, but you can expect the volume of fraction 1 to be the largest, followed by smaller volumes for fractions 2 and 3.
For more details about distillation click here:
https://brainly.com/question/21765936#
#SPJ11
Related Questions
Question 11 (1 point)
Scientists use comparative embryology to look into evolutionary relationships between animals, specifically those that look very
different from each other when they are fully formed.
True
False
Review Answers
Saved at 9:10 am
+ → X
Answers
Unlike solids and liquids, gases can be compressed. How is this physical characteristic of gases beneficial to society?
Answers
i need to know the answer ASAP PLEASE
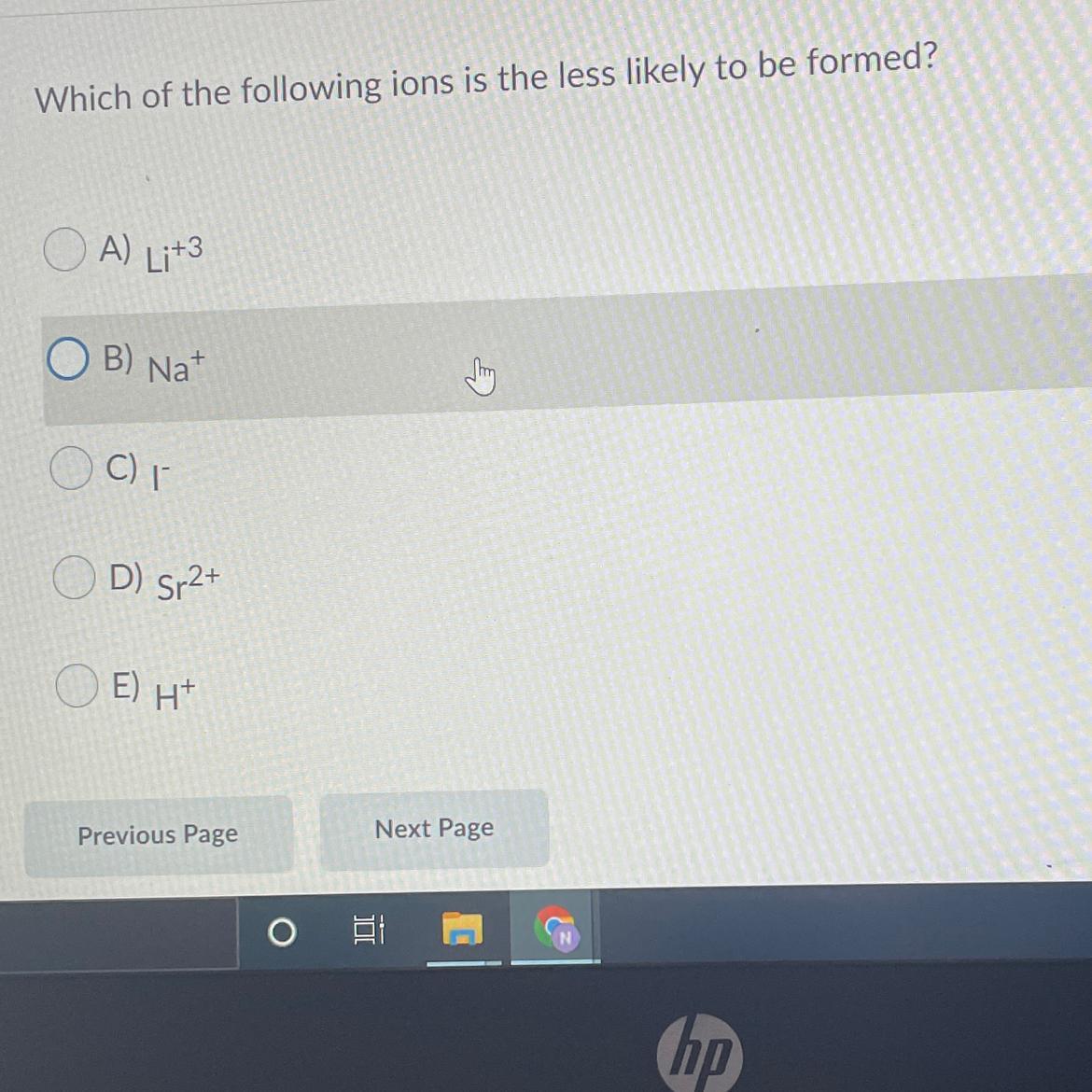
Answers
Answer:
E....H+
Explanation:
coz when hydrogen ions are formed they automatically join to form hydrogen
How do I identify the reducing agent in a redox reaction
Answers
Answer:
If you can identify the species that was being oxidized, its species that contains the element on the other side is the reducing agent.
For example, in the equation Zn + Cu2+ --> Zn2+ + Cu, the zinc was oxidized, making Zn the reducing agent.
What is the mole of 2 H2O?.
Answers
Answer:
2H2O have water = 2×2=4moles.
Explanation:
(◕ᴗ◕✿)(◕ᴗ◕✿)(◕ᴗ◕✿)
How might atomic radius have any influence on electronegativity trends or no influence on ionizatation
Answers
This is because electrons are drawn closer to protons, which have opposite charges and hence cling to them, in a small-radius atom.
If the radius is bigger, the electrons on the outside edge of the atom are not as tightly bound and are therefore more easily lost, requiring less energy to ionize.
Factors are more shielding (from core electrons) in the lowest elements of a family, allowing electrons to escape more easily. For those who are currently in a period, the effective nuclear charge grows as the period progresses (more protons, but no more energy levels, so the electrons are the same distance from the nucleus). This causes the electrons to be held closer together (smaller radius), requiring more energy to ionize them.
(a) Iron ore contains iron oxide.
Iron is extracted from iron oxide by heating the oxide with carbon.
(i) In this reaction
iron
oxide+ carbon—> iron +carbon dioxide
A carbon is reduced
B iron oxide is neutralised
C iron oxide is reduced
D iron is oxidised
A,b,c or d
Answers
In this reaction, the Iron oxide is reduced, and Carbon is the reducing agent. Hence, option C) Iron oxide is reduced is the correct answer.
The given reaction is written as follows: Iron oxide + Carbon → Iron + Carbon dioxide.The given options are:A) Carbon is reducedB) Iron oxide is neutralizedC) Iron oxide is reducedD) Iron is oxidizedThe correct option is C) Iron oxide is reduced.How is Iron extracted from Iron oxide?Iron is extracted from Iron oxide through reduction. A reducing agent is used to reduce Iron oxide to Iron. The most commonly used reducing agent is Carbon, which helps to convert Iron oxide to Iron. During the process of reduction, Carbon is oxidized to Carbon dioxide. The overall chemical reaction can be represented as follows:Fe2O3(s) + 3C(s) → 2Fe(s) + 3CO(g)The given reaction shows that Iron oxide is reduced to Iron, while Carbon is oxidized to Carbon dioxide.In this reaction, Iron oxide undergoes a reduction process because its oxidation state decreases, while Carbon undergoes an oxidation process because its oxidation state increases.
for such more questions on reaction
https://brainly.com/question/11231920
#SPJ8
2
Which represents a mixture?
*
Ос.
Answers
Explanation:
A mixture is an impure substances with the following properties:
Composition is indefinite i.e they consist of two or more elements and or compounds in any proportion by massConstituents retain their identities i.e physical properties are retainedConstituents reacts differently to changed conditions. They can easily be separated into constituents by physical methodsExamples are air, sea - water, muddy water, palm -wine, petroleum, alloys etc.
Using this description it should be easy to identify a mixture.
Which best demonstrates an example of the common ion effect?
duction
A The compound CuCl is 500 times less soluble in sea water than it is in
pure water.
ту
B Ammonia is produced commercially when an increase in pressure
shifts the equilibrium toward the product.
ompounds
C A lighted splint goes out in air but bursts into flame when plunged into
pure oxygen.
D The decomposition of carbonic acid in water occurs spontaneously
whereas the combination of carbon dioxide and water does not.
Answers
Answer: A. The compound CuCl is 500 times less soluble in sea water than it is in pure water.
pure water.
Explanation:
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle.
This principle states that if there is any change in a equilibrium reaction, the equilibrium will shift in a direction so as to minimize the effect.
Thus when a common ion is introduced to an equilibrium reaction, the equilibrium will shift in a direction where the concentration of common ion is decreasing.
\(CuCl\rightleftharpoons Cu^++Cl^-\)
\(NaCl\rightarrow Na^++Cl^-\)
When common ion such as \(Cl^-\) from NaCl in sea water is introduced to an equilibrium reaction, the equilibrium will shift in a direction where the concentration of common ion is decreasing i.e. in the left side and thus solubility of CuCl further decreases.
Objective: Utilize units (dimensional analysis) as a guide to problem solving. Apply the concepts of mole
and stoichiometry to aqueous solutions and gases.
Question: For the reaction SrCO (s) + 2HCl(aq) → SrCl₂(aq) + CO₂(g) + H₂O(l), what volume of carbon
dioxide gas, measured at 1.05 atm and 298 K, may be produced by the reaction of 25,0 mL of 0.1072 M
HCI with excess strontium carbonate?
Include definitions, explanations, discussion of problem-solving process.
Answers
The volume of carbon dioxide gas produced will be 23.3mL
SrCO (s) + 2HCl(aq) → SrCl₂(aq) + CO₂(g) + H₂O(l)
Here concentration of the strontium carbonate is in excess and the concentration of HCl is 25mL having molarity 0.1072 M
Number of moles of HCl= mass/ molar mass,
Molar mass of HCl= mass/ Number of moles
Molar mass of HCl= 36.5
Mass of HCl from balanced equation= 2×36.5=73
Molarity= Number of moles/ volume
According to stoichiometry the moles of HCl is 2 and that of CO₂ is 1.
We know that,
PV =nRT
1.05× V= 1 ×0.0821× 298
V= 0.0821×298/ 1.05
= 23.3 mL
By ideal gas equation, volume of carbon dioxide is calculated as 23.3 mL.
To know more about stoichiometry, please refer:
https://brainly.com/question/29042675
#SPJ1
Predict whether the entropy change for this process is positive or negative and explain your answer. Then, predict whether the free energy change for the process is positive or negative and explain your answer.
Answers
The given question is incomplete. The complete question is:
Consider the spontaneous dissolution of NaCl in water: NaCl(s) → Na+(aq) + Cl −(aq) Predict whether the entropy change for this process is positive or negative and explain your answer. Then, predict whether the free energy change for the process is positive or negative and explain your answer.
Answer: The entropy change is positive. The free energy change for the process is negative and reaction is spontaneous.
Explanation:
Entropy is defined as the degree of randomness of a system. For a system in which randomness increses , the enetropy is said to increase and \(\Delta S\) is positive.
As in the given reaction : \(NaCl(s)\rightarrow Na^+(aq)+Cl^-(aq)\) , the solid is dissociating to give ions , randomness increases and thus entropy is positive.
Also the dissociation of a molecule requires energy , thus the enthalpy \(\Delta H\) is also positive as heat is absorbed by the system.
\(\Delta G=\Delta H-T\Delta S\)
\(\Delta G=(+ve)-T(+ve)\)
\(\Delta G=-ve\) when Temperature is high.
Consider the spontaneous dissolution of NaCl in water: NaCl(s) → Na+(aq) + Cl −(aq)
Predict whether the entropy change for this process is positive or negative and explain your answer. Then, predict whether the free energy change for the process is positive or negative and explain your answer.
The given question is incomplete. The complete question is:
Consider the spontaneous dissolution of NaCl in water: NaCl(s) → Na(aq) Cl −(aq) Predict whether the entropy change for this process is positive or negative and explain your answer. Then, predict whether the free energy change for the process is positive or negative and explain your answer.
Answer: The entropy change is positive. The free energy change for the process is negative and reaction is spontaneous.
Explanation:
Entropy is defined as the degree of randomness of a system. For a system in which randomness increses , the enetropy is said to increase and is positive.
As in the given reaction : , the solid is dissociating to give ions , randomness increases and thus entropy is positive.
Also the dissociation of a molecule requires energy , thus the enthalpy is also positive as heat is absorbed by the system.
when Temperature is high.
Which of these ideas did you include in your answer?
✔️ When a crystalline solid dissolves, it becomes more disordered.✔️ When disorder increases, ΔG is positive.✔️ This process probably has a positive ΔS .✔️ The process is spontaneous.✔️ Spontaneous processes have ΔG < 0; the process has a negative ΔG .
Have a Nice Day .
Water and sand are being separated by filtration. What physical change is used?
Answers
Felting is a physical procedure. The mixture of the given solid and the liquid can be separated using filter paper in this process, for example, sand and water can be separated using filter paper.
What is filtration ?Filtration is the process of removing solid particles from a liquid or gaseous fluid by using a filter medium that allows the fluid to pass through but retains the solid particles. The desired product could be either the clarified fluid or the solid particles removed from the fluid.
Separating sand from water is a physical process rather than a chemical one. Sand and water are a mixture that can be easily separated without the use of any chemicals. Sand can be filtered out of the water with a sieve and then dried to allow any remaining water to evaporate.
Thus,Water and sand are being separated by filtration.Felting is used as a physical change.
To learn more about the filtration, follow the link;
https://brainly.com/question/29056566
#SPJ1
in which part of the sun would the most helium be found?
Answers
it's most abundant in the mid latitudes.
Draw Lewis structure(s) for the carbonate ion (CO32-). If there are equivalent resonance structures, draw all of them.
Answers
The Lewis structure for the carbonate ion (CO32-) can be drawn by first identifying the valence electrons of each atom and arranging them to form bonds and fulfil the octet rule. Carbon has 4 valence electrons, while each oxygen atom has 6. This gives a total of 22 valence electrons for CO32-.
To begin, we can place a single bond between each oxygen atom and the carbon atom. This uses up 6 electrons (2 from each bond), leaving 16 remaining. We can then place two lone pairs on each oxygen atom, which uses up an additional 12 electrons (6 from each pair), leaving 4 remaining. These remaining electrons can be placed as a lone pair on the central carbon atom. This gives us the following Lewis structure for the carbonate ion:
O
//
O C
\\
O-
However, this is not the only way that the electrons can be arranged in the molecule. There are actually two equivalent resonance structures that can be drawn for CO32-. To draw these, we can move one of the lone pairs from an oxygen atom to form a double bond with the adjacent oxygen atom. This gives us the following structures:
O- O
/ ||
O C <--> O=C=O
\\ ||
O O-
Both of these structures are equivalent in terms of their overall electronic structure. They are also important for understanding the bonding in the carbonate ion, as the true structure of the molecule is likely a combination of these resonance structures.
Learn more about Lewis structure here ;
https://brainly.com/question/29756546
#SPJ11
Which of the following is NOT a feature that supports the particulate theory of matter?
A. There are empty spaces between the particles
B. The particles are in constant motion
C. There are no forces of attraction between the particles
D. Temperature has an effect on the speed of motion of the particles
Answers
There are no forces of attraction between the particles.
The kinetic energy of the molecule is greater than the attractive force between them, thus they are much farther apart and move freely from each other. In most cases, there are essentially no attractive forces between particles. This means that gas has nothing to hold a specific shape or volume.
Particles
In the physical sciences, a particle (or corpuscle in older texts) is a small localized object to which can be ascribed several physical or chemical properties, such as volume, density, or mass. They vary greatly in size or quantity, from subatomic particles like the electron to microscopic particles like atoms and molecules, to microscopic particles like powders and other granular materials. Particles can also be used to create scientific models of even larger objects depending on their density, such as humans moving in a crowd or celestial bodies in motion.
The term particle is rather general in meaning and is refined as needed by various scientific fields. Anything that is composed of particles may be referred to as particulate. However, the noun particulate is most frequently used to refer to pollutants in the Earth's atmosphere, which are a suspension of unconnected particles, rather than a connected particle aggregation.
Learn more about Particles
https://brainly.com/question/18826360
#SPJ2
Question 5 of 10
Two hydrogen atoms combine chemically with one oxygen atom to form
water. What is water?
O A. An atom
B. A molecule
O C. An element
O D. A mixture
Answers
At terminal velocity, which two forces are balanced? *
gravity and air resistance
gravity and magnetism
O magnetism and air resistance
O friction and air resistance
Answers
Answer:
friction and air resistance
at terminal velocity, the weight of the object due to gravity is balanced by the frictional forces, and the resultant force is zero.
When two or more different elements bond together, compounds are formed. The smallest parts of these compounds that retain their original properties are called molecules. All atoms contain negatively charged electrons that orbit around a positively charged nucleus. The nucleus contains positively charged ____________ and neutrally charged ____________ . It is the ____________ charged ____________ that give atoms properties that are favorable in forming chemical bonds.
Answers
Answer:
protons, neutrons, negatively, electrons
Explanation:
protons are positive, neutrons are neutral. the entire basis of bonding is chemistry is the distribution of electrons, which are negatively charged particles surrounding the nucleus.
Determine the mass of 10g of CaCO3
Answers
Answer:
= 100u. Hence 10 g = 0.1 mole. Hope it's helpful to u
For the elementary reaction NO3 + CO ❝ NO2 + CO2 the molecularity of the reaction is __________, and the rate law is rate = __________. A) 2, k[NO3][CO] B) 4, k[NO3][CO][NO2][CO2] C) 2, k[NO2][CO2] D) 2, k[NO3][CO]/[NO2][CO2] E) 4, k[NO2][CO2]/[NO3][CO].
Answers
For the elementary reaction NO3 + CO ❝ NO2 + CO2 the molecularity of the reaction and the rate law is 2 and k[NO3][CO] respectively. Therefore, the correct option is A) 2, k[NO3][CO]
The quantity of responding molecules that collide simultaneously to produce a chemical reaction is known as the molecularity of a reaction. A chemical reaction's rate and the concentrations of the reactants involved are correlated by an expression known as the rate law, commonly referred to as the rate equation.
The molecularity of the reaction is 2 because there are two reactant molecules involved in the elementary reaction.
The rate law is rate = k[NO3][CO] because the rate of the reaction depends on the concentration of both reactants. Therefore, the correct answer is A) 2, k[NO3][CO].
Learn more about molecularity: https://brainly.com/question/8327819
#SPJ11
What does it mean to neutralize a chemical solution?
Answers
Answer:
A neutralized solution in chemistry refers to the reaction between an acid and base that results in a neutral balance, or a measure of 7 on the pH scale.
Explanation:
at 25 °c, how many dissociated h ions are there in 325 ml of an aqueous solution whose ph is 11.41? number of h ions:
Answers
Aat 25 °C, the number of dissociated H+ ions there are in 325 ml of an aqueous solution whose pH is 11.41 is 1.235 × 10⁻¹².
The pH scale is a logarithmic measure of the acidity or basicity of an aqueous solution. The pH scale ranges from 0 to 14, with 7 being neutral, below 7 being acidic, and above 7 being basic or alkaline. Therefore, we can use the relationship between pH and [H+] concentration to calculate the number of dissociated H+ ions in an aqueous solution. The relationship is as follows:
pH = -log[H+]
or [H+] = 10^-pH
We can use this relationship to find the number of dissociated H+ ions in 325 mL of an aqueous solution whose pH is 11.41 as follows:
First, we need to find the [H+] concentration from the given pH:
[H+] = 10^-pH = 10^-11.41 = 3.80 × 10⁻¹² M
Now that we know the [H+] concentration, we can calculate the number of dissociated H+ ions using the following formula:
Number of dissociated H+ ions = [H+] × volume of solution in Liters
The volume of the solution is given in milliliters (mL), so we need to convert it to liters (L):
325 mL = 325/1000 L = 0.325 L
Now we can use the formula above to find the number of dissociated H+ ions:
Number of dissociated H+ ions = [H+] × volume of solution in Liters = 3.80 × 10⁻¹² M × 0.325 L = 1.235 × 10⁻¹² mol
The answer is: 1.235 × 10⁻¹² dissociated H+ ions.
Learn more about pH scale here: https://brainly.com/question/26424076
#SPJ11
What is the boiling point of a solution made using 739 g of sucrose, C12H22O11, in 0.300 kg of water, H2O?
Answers
The boiling point of the sucrose solution would be 3.68 °C compared to the boiling point of pure water.
The boiling point of a solution depends on the concentration of solute particles in the solution, which affects the colligative properties of the solution, such as boiling point elevation.
To calculate the boiling point elevation, we can use the following formula;
ΔTb = Kbm
where ΔTb is the boiling point elevation, Kb is the molal boiling point constant for water (which is a constant value), and bm is the molality of the solution, which is the amount of solute (in moles) per kilogram of solvent.
First, let's calculate the molality of the solution;
moles of sucrose = mass of sucrose / molar mass of sucrose
moles of sucrose = 739 g / 342.3 g/mol (molar mass of sucrose)
Next, let's convert the mass of water to kilograms:
mass of water = 0.300 kg
Now, we can calculate the molality of the solution;
bm = moles of sucrose / mass of water
Plugging in the values;
moles of sucrose = 739 g / 342.3 g/mol ≈ 2.158 mol
mass of water = 0.300 kg
bm = 2.158 mol / 0.300 kg ≈ 7.193 mol/kg
Next, we need to determine the molal boiling point constant (Kb) for water. The molal boiling point constant for water is approximately 0.512 °C kg/mol.
Finally, we can calculate the boiling point elevation;
ΔTb = Kb × bm
ΔTb = 0.512 °C kg/mol × 7.193 mol/kg
≈ 3.68 °C
To know more about boiling point here
https://brainly.com/question/25777663
#SPJ1
What could J. J. Thomson conclude from his experiments?
Answers
Answer:
He concluded that atoms contain small negatively charged particles that are called electrons.
How many atoms are in 9.35 moles of lithium?
Answers
There are approximately 5.621 x 10^24 lithium atoms in 9.35 moles of lithium.
To determine the number of atoms in 9.35 moles of lithium, we can use Avogadro's number, which is the number of particles (atoms, molecules, etc.) in one mole of a substance. Avogadro's number is approximately 6.022 x 10^23 particles per mole.
First, we need to calculate the total number of lithium atoms in 9.35 moles of lithium:
Number of lithium atoms = (9.35 mol) x (6.022 x 10^23 atoms/mol)
Number of lithium atoms = 5.621 x 10^24 atoms
In chemistry, a mole is a unit of measurement used to express the amount of a substance. One mole of a substance is defined as the amount of the substance that contains the same number of particles as there are atoms in 12 grams of pure carbon-12. This number is known as Avogadro's number and is approximately 6.022 x 10^23 particles per mole.
For more question on lithium atoms click on
https://brainly.com/question/30377436
#SPJ11
Which of the following is the correct definition of chemical energy?
A.
energy stored in chemical bonds of molecules
B.
energy produced from the splitting of atoms
C.
energy of an object due to the random motion of its atoms and molecules
D.
energy an object has because of its motion or position
PLSHEL HELP DUE TODAY
Answers
Answer:
A
Explanation:

A lake that is used as a water source receives a lot of acid rain one year. Which of the following is an impact this will have?(1 point)
The lake water will have a lower pH which will cause high levels of fog in the area, making it hard to see.
The lake water will have a higher pH which will make it harder for plants round the lake to access nutrients.
The lake water will have a higher pH which will damage the boats that people store on the docks on the lake.
The lake water will have a lower pH which will damage the plants and animals living in the water.
Answers
The lake water will have a lower pH which will damage the plants and animals living in the water is the impact the acid rain will have on the lake and is denoted as option D.
What is Acid rain?This is the type of rain which has acidic properties due to the increase in the number of hydrogen ions and is common in industrial areas where chemicals are used for their operation.
This type of rain have different effects on water bodies such as lakes such that the lowering of the pH leads to the damage of the plants and animals living in the water.
Read more about Acid rain here https://brainly.com/question/718250
#SPJ1
Answer: water
Explanation: 123
suppose that the mass you determined for your final piece of data, the mass of crucible plus copper sulfide a er second heating, was 0.002 g too low. what would be the percentage of copper calculated? would this error change the empirical formula you would report? explain. 5. calculate the uncertainty in the subscripts in the formula you found in this experiment. not for distribution - for instructorsors useeeeee ononononononononlylylylylylylylylylyly
Answers
The percentage of copper that would be calculated would be 0.002 g lower than it should have been. This error would not change the empirical formula reported, as the error is a result of an incorrect mass measurement and not incorrect chemical formula or other factors of the empirical formula.
What is empirical formula?The definition of an empirical formula for a compound is one that displays the ratio of the elements present in the compound but not the precise number of atoms in the molecule. Subscripts are used next to the element symbols to indicate the ratios. The subscripts in the empirical formula, which represent the ratio of the elements, are the smallest whole numbers, making it also known as the simplest formula.
How to calculate empirical formula?1) Start by listing the gram of each element, which you often find in an experiment or are required to provide in a question.
2)To make the computation easier, assume the entire mass of a sample is 100 gram, so you can work with simple percentages. Set the mass of each element to the percent, in other words. The total should be 100 percent.
3) You may convert the mass of each element in the periodic table into moles by multiplying its atomic weight by its molar mass.
4)Multiply each mole value by the few moles you determined through computation.
5) To the nearest whole number, round each number you receive.
To know more about empirical formula, visit:
https://brainly.com/question/14044066
#SPJ1
calculate the ph of a buffer containing 0.18 m h2co3 and 0.25 m nahco3. the ka of h2co3 at 25 °c is 4.3 × 10−7.
Answers
The pH of the buffer solution containing 0.18 M H₂CO₃ and 0.25 M NaHCO₃ is approximately 6.513.
To calculate the pH of the buffer solution, we can use the Henderson-Hasselbalch equation;
pH = pKa + log([A⁻]/[HA])
In this case, H₂CO₃ acts as a weak acid (HA) and its conjugate base, HCO₃⁻, acts as the salt (A⁻).
Given; [H₂CO₃] = 0.18 M
[HCO₃⁻] = 0.25 M
pKa = -log(Ka) = -log(4.3 × 10⁻⁷)
Let's substitute the values into the Henderson-Hasselbalch equation and calculate the pH;
pH = (-log(4.3 × 10⁻⁷) + log(0.25/0.18)
pH = (-(-6.37)) + log(1.39)
pH = 6.37 + 0.143
pH = 6.513
Therefore, the pH of the buffer solution will be 6.513.
To know more about buffer solution here
https://brainly.com/question/30332096
#SPJ4
Explain how copper is produced from copper() sulfate solution by electrolysis?
Answers
Answer: Copper is purified by electrolysis . Electricity is passed through solutions containing copper compounds, such as copper(II) sulfate. The anode (positive electrode ) is made from impure copper and the cathode (negative electrode) is made from pure copper. Pure copper forms on the cathode.