What is the reaction and product of each of the formulas
HCI + NaOH --> H₂O + NaCl
_C3H8 + 502 --> 3CO₂ + 4H₂O
2Li+F2 --> 2LiF
2AgNO3 + CuCl2 --> _Cu(NO3)2 + 2AgCl
Answers
Explanation:
1. HCI + NaOH --> H₂O + NaCl is a neutralisation reaction. The product is soluble sodium chloride salt and water.
2. _C3H8 + 502 --> 3CO₂ + 4H₂O is a propane combustion reaction. The product is carbon dioxide gas and water.
4. 2Li+F2 --> 2LiF is a combination reaction. The product is insoluble lithium fluoride solid
5.2AgNO3 + CuCl2 --> _Cu(NO3)2 + 2AgCl is a precipitation reaction. The product is copper(II) nitrate and an insoluble silver chloride salt(precipitate)
Related Questions
How many grams of copper nitrate can be produced from 0.78 grams of silver nitrate and excess copper?
Answers
Answer:
Explanation:
This link will take you to a work sheet that I think might help.
In a food chain, energy does NOT flow directly from - F producer to decomposer G producer to consumer H consumer to decomposer J consumer to producer
Answers
Answer:
producer to decomposer
Explanation:
This is because in a food chain , energy flow from one trophic level to another. The producer which include plants are the source of energy which they manufacture good in the presence of light energy from sun. Energy flow directly from the producer to the primary consumer which are heterotrophs that feed on plants. Energy flow from consumer to decomposer after the consumer died and it is decayed.
Which of the following does NOT normally exist as a diatomic molecule? *
PLS HELP ME I NEED HELP
Oxygen gas
Chlorine gas
Helium gas
Nitrogen gas
Hydrogen gas
Answers
Answer:
Helium gas
Explanation:
a method you could use to remember the Diatomic molecules is HOFBrINCl
which stands for all of the Diatomic molecules and as you could observe Helium is not included!
Answer:
Helium gas
Explanation:
because it's helium :/
how many particles would be found in 1.224 g sample of K2O?
Answers
Answer:
7.8286×10^21 particles
Explanation:
atomic mass of potassium = 39.1 u
1 u = 1 g/mol
Potassium (K) = 39.1 g/mol × 2 = 78.2 g/mol
Oxygen (O) = 16 g/mol × 1 = 16 g/mol
K₂O = 94.2 g/mol
1.224 g / 94.2 g/mol = .013 moles
Avogadro's number = 6.022×10²³
particles = moles x Avogadro's number
particles = .013 moles x 6.022×10^23
particles = 7.8286×10^21 particles
https://brainly.com/question/12867103
socratic
How does extreme pressure affect the volume occupied by a real gas compared to the predictions of the ideal gas law? (3 points)
Group of answer choices
The true volume of the gas will be higher than predicted by the ideal gas law because the attraction between the particles is weaker under high pressure.
The true volume of the gas will be lower than predicted by the ideal gas law because the attraction between the particles is weaker under high pressure.
The true volume of the gas will be higher than predicted by the ideal gas law because the volume of the actual particles is more significant under high pressure.
The true volume of the gas will be lower than predicted by the ideal gas law because the volume of the actual particles is more significant under high pressure.
Answers
At a very high pressure, the volume of occupied by a real gas is greater than the volume predicted by the ideal gas law, because the volume of the actual particles is more significant under high pressure.
Behavior of gases at low pressure and high pressuresAt low pressure, the distance between gas molecules is relatively far apart, but as the pressure of the gas increases, the distances between theses gaseous molecules becomes smaller.
Due to the high pressure the volume occupied by the gas molecules becomes significant compared with the volume of the container.
As a result, the total volume occupied by the gas will be greater than the volume predicted by the ideal gas law.
Thus at a very high pressure, the volume of occupied by a real gas is greater than the volume predicted by the ideal gas law, because the volume of the actual particles is more significant under high pressure.
Learn more about behavior gases under pressure here: https://brainly.com/question/13512523
Answer: The fourth answer
Explanation:
Under high pressure, real gases deviate from ideal gas behavior as their particles have a finite size and experience intermolecular forces, causing the actual volume of the gas to be lower than predicted by the ideal gas law.
Which type of molecule does not occur naturally in animals or plants and is found in many processed foods?
Answers
Artificial additives are a type of molecule that does not occur naturally in animals or plants but is found in many processed foods.
Artificial additives are substances added to processed foods to enhance their flavor, color, texture, or shelf life. These additives are typically created through chemical processes and are not naturally found in the ingredients used in animal or plant-based foods.Common examples of artificial additives include artificial sweeteners, artificial food coloring, preservatives, and flavor enhancers. While they serve various purposes in processed foods, it is important to note that their presence in such foods is not a natural occurrence in animals or plants.
To learn more about Artificial additives:
https://brainly.com/question/13166331
#SPJ11
This ether can, in principle, be synthesized by two different combinations of haloalkane and metal alkoxide. Draw the combination of alkyl chloride and potassium alkoxide that forms the higher yield of ether.
Answers
To form the higher yield of ether, we need to consider the reactivity of the haloalkane and the metal alkoxide. In general, alkyl chlorides are less reactive compared to alkyl bromides and alkyl iodides.
Potassium alkoxides, on the other hand, are more reactive compared to sodium and lithium alkoxides. Therefore, to achieve a higher yield of ether, we can use a more reactive haloalkane and a more reactive metal alkoxide.
One example of a combination that can form a higher yield of ether is the reaction between tert-butyl chloride (a more reactive haloalkane) and potassium tert-butoxide (a more reactive metal alkoxide).
The reaction can be represented as follows:
CH33CCl + KOt-Bu -> CH3CCOt-Bu + KCl
In this reaction, tert-butyl chloride reacts with potassium tert-butoxide to form tert-butyl tert-butoxide, which then rearranges to form the desired ether. This combination is more favorable as it involves more reactive compounds, leading to a higher yield of ether.
It's important to note that there may be other combinations that can also result in a high yield of ether. The specific choice of haloalkane and metal alkoxide will depend on their reactivity and the desired product. Always refer to the reactivity series and consider the properties of the compounds involved when selecting the combination for synthesis.
To know more about alkyl iodides visit:-
https://brainly.com/question/31555985
#SPJ11
Every atom of the __ carbon has six protons
Answers
The angle formed by the nuclei of two surrounding atoms with the nucleus of the central atom in a structure is called a(n) ________angle. The value predicted for such an angle using the VSEPR theory would be 180 degrees, based upon geometry alone. This is referred to as the___________ bond angle. In practice, this value often deviates from the predicted value for various reasons.
Answers
The bond angle obtained based on the geometry of the molecule alone is known as the predicted bond angle.
When a compound is formed, the less electronegative atom in the compound is called the central atom in the molecule. The angle formed by the nuclei of two surrounding atoms with the nucleus of the central atom in a structure is called a bond angle.
The bond angle is usually based on the geometry of the molecule. The expected geometry can be deviated for certain reasons such as the presence of lone pairs on the central atom in the molecule. The bond angle obtained based on the geometry of the molecule alone is known as the predicted bond angle.
Learn more about bond angle: https://brainly.com/question/6179102
Four different living organisms are shown below. Which statement is true for all of the organisms shown? The organisms are an ameba rabbit human and tree
Answers
12. What is the mass number of an atom that has 4 protons, 4 electrons, and 5 neutrons?
Answers
Answer:
9
Explanation:
mass number = protons + neutrons
= 4 + 5 = 9
CH4 (g)+ 202(g)=CO2(g) + 2H2O(l)If 133.2 grams of CH4 react with excess O2 , how many grams of H2O result?
Answers
299.7g of H2O will be produced.
1st) With the balanced chemical equation and the molar mass of CH4 and H2O, we can calculate the amount of H2O that is produced by stoichiometry:
- CH4 molar mass: 16g/mol
- H2O molar mass: 18g/mol
According to the balanced equation, 16g of CH4 will produce 36g of H2O (2 x 18g).
2nd) Now, with a mathematical rule of three we can calculate the grams of water that will be produced from 133.2 grams of CH4:
\(\begin{gathered} 16gCH_4-36gH_2O \\ 133.2gCH_4-x=\frac{133.2gCH_4\cdot36gH_2O}{16gCH_4} \\ x=299.7gH_2O \end{gathered}\)Finally, 299.7g of H2O will be produced from 133.2 grams of CH4.
Can someone help me with this
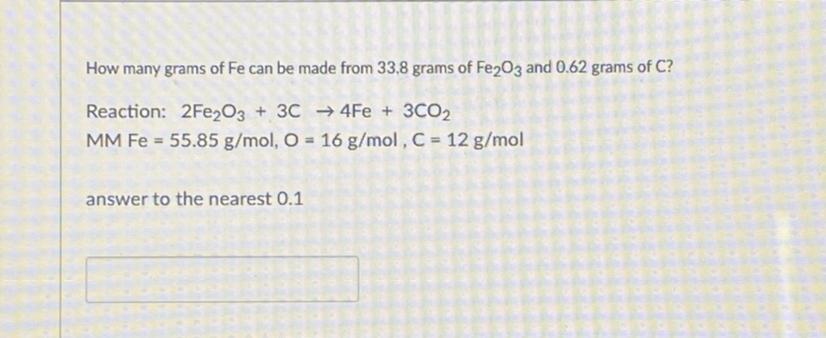
Answers
When an electron move from a low energy level to a high energy, it ______ energy.
Answers
explained?
When an electron moves from a lower energy level to a higher energy level, energy is absorbed by the atom. When an electron moves from a higher to a lower energy level, energy is released (often as light).
At room temperature, an element that is a brittle solid and a poor conductor of
heat and electricity could have an atomic number of
Answers
solo necesito puntos lo siento
Explanation:
How many milliseconds (ms) are there in 3. 5 seconds (s)? 35 ms 350 ms 3,500 ms 35,000 ms.
Answers
Measurement units are the defined units used for the particular dimensions and proportions. 3500 milliseconds are there in seconds.
What is the unit of time?Time is defined as the rate of things occurring or taking place and is given by seconds, minutes, hours etc.
Using the conversion factor the relation between the seconds and milliseconds is:
1 second = 1000 miliseconds
Convert 3.5 seconds into milliseconds using conversion factor:
\(\begin{aligned}3.5 \;\rm seconds &= 3.5 \times 1000 \\\\&= 3500 \;\rm miliseconds\end{aligned}\)
Therefore, option C. 3500 milliseconds is correct.
Learn more about time and second here:
https://brainly.com/question/2025518
Answer:
3500 ms
Explanation:
What is weight?
1. It is a measure of how much material an object contains.
2. It is a measure of the force of gravity pulling down on an object.
3. It is a type of object with a unusual shape.
4. It is the amount of space that matter takes up.
Answers
Answer:
2
the rest doesnt make sense
Convert 22.7 grams of H2O to molecules
Answers
Answer: 1.2600414759026783 mol
Explanation:
Please can someone help I will mark brainiest!

Answers
Answer:
2Cl- ⇒ Cl ↓2+ 2e
Explanation: sorry if this is not what you were looking for.
calcium fluoride, caf2, has a molar solubility of 2.1 10–4 mol l–1 at ph = 7.00. by what factor does its molar solubility increase in a solution with ph = 3.00? the pka of hf is 3.17.
Answers
Answer:
First, we need to determine the effect of changing the pH on the solubility of calcium fluoride. Since calcium fluoride is a salt of a weak acid (HF) and a strong base (CaOH), we can use the common ion effect to calculate the change in solubility.
At pH = 7.00, the concentration of HF is:
[H+] = 10^(-pH) = 10^(-7) = 1.0 x 10^-7 M
Ka = 10^(-pKa) = 10^(-3.17) = 7.9 x 10^-4
[H+] [F-]/[HF] = Ka
[F-]/[HF] = Ka/[H+] = (7.9 x 10^-4)/(1.0 x 10^-7) = 7.9 x 10^3
[S] = [CaF2] = 2.1 x 10^-4 M
Using the common ion effect, we can calculate the new concentration of fluoride ions (F-) at pH = 3.00.
[H+] = 10^-pH = 10^-3 = 1.0 x 10^-3 M
[F-]/[HF] = Ka/[H+] = (7.9 x 10^-4)/(1.0 x 10^-3) = 0.79
[F-] = [HF](0.79) = (2.1 x 10^-4)(0.79) = 1.66 x 10^-4 M
Therefore, the new molar solubility of calcium fluoride at pH = 3.00 is:
[S] = [CaF2] = [F-] = 1.66 x 10^-4 M
The factor by which the molar solubility increases is:
(1.66 x 10^-4 M)/(2.1 x 10^-4 M) = 0.79
Therefore, the molar solubility of calcium fluoride increases by a factor of 0.79 in a solution with pH = 3.00.
The molar solubility of caf2 increases by a factor of 1.39 in a solution with pH = 3.00 compared to a solution with pH = 7.00, due to the presence of F- ions from the dissociation of HF.
The solubility of calcium fluoride, caf2, is affected by the pH of the solution. At pH 7.00, the molar solubility of caf2 is 2.1 x 10-4 mol/L. To determine how the molar solubility changes at pH 3.00, we need to consider the acid-base equilibrium of the solution.
The pKa of HF is 3.17, which means that at pH 3.00, HF will be mostly in its protonated form, H2F+. The presence of H2F+ will decrease the solubility of caf2. We can use the common ion effect to calculate the new molar solubility of caf2 at pH 3.00.
First, we need to calculate the concentration of fluoride ions, F-, in the solution at pH 3.00. At this pH, most of the HF will be in its protonated form, H2F+, and only a small fraction will be in its deprotonated form, F-. The concentration of F- can be calculated using the Henderson-Hasselbalch equation:
pH = pKa + log([F-]/[HF])
Rearranging the equation gives:
[F-]/[HF] = 10pH-pKa
Substituting the values of pH and pKa gives:
[F-]/[HF] = 10^(-0.17) = 0.426
The concentration of F- is therefore 0.426 times the concentration of HF.
Next, we can use the solubility product constant, Ksp, of caf2 to calculate its molar solubility in the presence of F-. The expression for Ksp is:
Ksp = [Ca2+][F-]2
At equilibrium, the concentration of Ca2+ is equal to the molar solubility of caf2, so we can substitute the value of Ksp and the concentration of F-:
Ksp = [caf2] [F-]2
2.87 x 10^-10 = [caf2] (0.426)^2
Solving for [caf2] gives:
[caf2] = 2.91 x 10^-4 mol/L
Therefore, the molar solubility of caf2 increases by a factor of:
(2.91 x 10^-4 mol/L) / (2.1 x 10^-4 mol/L) = 1.39
The molar solubility of caf2 increases by a factor of 1.39 in a solution with pH = 3.00 compared to a solution with pH = 7.00, due to the presence of F- ions from the dissociation of HF.
Learn more about molar solubility here
https://brainly.com/question/28170449
#SPJ11
HELP ME ASAPPP plsss

Answers
Which statement describes the redox reaction that occurs when an object is electroplated? * 1 point A. It is spontaneous and requires an electric current. B. It is spontaneous and produces an electric current. C. It is nonspontaneous and requires an electric current. D. It is nonspontaneous and produces an electric current.
Answers
Answer:
C. It is nonspontaneous and requires an electric current.
Explanation:
Electroplating refers to the process of coating a metal on the surface of another metal. Electroplating may be carried out to improve the properties of a metal or to preserve it from corrosion.
Electroplating involve the process of electrolysis. In electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. Electrolytes are decomposed during electrolysis by the passage of direct current to form ions.
The entire process of electrolysis is non spontaneous. It requires the input of electric electric current. This is achieved by connecting a battery to supply energy to drive the non spontaneous process.
What is meant by "the energy of an electron is quantized"?
The quantity of electron energy can be measured.
Each electron around an atom has a discrete amount of energy.
The quantity of electron energy changes as it moves around the nucleus.
all of the above
Answers
Answer:
d: All of the above. Have a nice day!
Answer:
The answer is: All of the above
Explanation:
the boiling point of liquid b is 95.5 °c at 1 atm (760.0 torr). its vapor pressure at 73.5 °c is 315 torr. what is its ∆hvap in kj/mol?
Answers
The enthalpy of vaporization (∆Hvap) of liquid B is approximately 16.89 kJ/mol.
What is enthalpy?To calculate the enthalpy of vaporization (∆Hvap) in kJ/mol, we can use the Clausius-Clapeyron equation. The equation relates the vapour pressure of a substance at one temperature to its vapour pressure at another temperature and the enthalpy of vaporization. The equation is as follows:
ln(P₂/P₁) = -∆Hvap/R x (1/T₂ - 1/T₁)
Where:
P₁ and P₂ are the vapour pressures at temperatures T₁ and T₂, respectively.
∆Hvap is the enthalpy of vaporization.
R is the ideal gas constant (8.314 J/(mol·K)).
Converting the given temperatures and pressures to Kelvin and Pascal, respectively, we have:
T₁ = 73.5 °C + 273.15 = 346.65 K
P₁ = 315 torr x (101325 Pa / 760 torr) = 41842.76 Pa
T₂ = 95.5 °C + 273.15 = 368.65 K
P₂ = 760.0 torr x (101325 Pa / 760 torr) = 101325 Pa
Substituting these values into the Clausius-Clapeyron equation:
ln(101325/41842.76) = -∆Hvap/(8.314) x (1/368.65 - 1/346.65)
Simplifying the equation:
ln(2.423) = -∆Hvap/(8.314) x (0.0027205 - 0.002885)
ln(2.423) = -∆Hvap/(8.314) x (-0.0001645)
Now, we can solve for ∆Hvap:
-∆Hvap/(8.314) = ln(2.423) / (-0.0001645)
∆Hvap = -8.314 x (ln(2.423) / (-0.0001645))
Calculating the value:
∆Hvap ≈ -8.314 x (-2.0327) = 16.89 kJ/mol
Therefore, the enthalpy of vaporization (∆Hvap) of liquid B is approximately 16.89 kJ/mol.
To know more about enthalpy follow
https://brainly.com/question/28300561
#SPJ6
below given are some example of solution identify solute and solvent from them also mention whether they are solid, liquid and gas . Air,milk,water, pepsi,humid air,sea water, polluted air
Answers
Answer:
Solutes examples are
Air,(gas) humid air (gas), polluted air(gas)
Examples of Solvents are Milk(liquid), water(liquid).,
Pepsi(liquid).
Sea water(liquid)
Explanation:
Solutes are substances that are dissolved and it is present in minute fraction in a solution.
Solvents is the medium that dissolve substances or solutes and it is present in large amount in a solution.
Solutes and solvents for solution.
Solution is formed when substances dissolves in another.
Air: The solvent is nitrogen because it is present in large quantities and the solutes is oxygen because it is present in small quantity.
Pepsi :sugar, caffeine and carbon dioxide as solutes and the solvent is water.
Milk: lactose, some minerals are solutes and Water is the solvent.
Humid air: water is the solutes and gases is the solvent.
Sea water: salt is the solute and water is the solvent.
Polluted air: Air is the solvent and dust particles is the solutes.
the fifth row of the periodic table corresponds to what energy level in the atom?
Answers
The fifth row of the periodic table corresponds to the fifth energy level (also known as the "n=5" energy level) in the atom.
In the context of atomic structure, each energy level represents a specific energy state or shell in which electrons can exist around the nucleus of an atom. The energy levels are labeled using a principal quantum number (n), with n = 1 corresponding to the first energy level (closest to the nucleus) and increasing with each subsequent energy level.
The fifth row of the periodic table consists of elements that have their outermost electrons occupying the fifth energy level. These elements have their valence electrons, which participate in chemical bonding and determine the element's chemical properties, located in the fifth energy level.
It's important to note that the periodic table is organized in a way that groups elements with similar electronic configurations and chemical properties together. Thus, elements in the same row generally have similar characteristics and exhibit periodic trends in their properties.
To know more about periodic table , refer here :
https://brainly.com/question/31672126#
#SPJ11
8. Which type of element requires the least amount of energy to remove an electron?
Answers
Answer:
nonmetals
Explanation:
how many moles of oxygen atoms are in one mole of the molecule Ca5(PO4)3OH? could you also show work
Answers
Answer:
13 moles of oxygen
Explanation:
A mole of a substance is the unit of measuring the number of particles within a chemical substance.
The given compound is:
Ca₅ (PO₄)₃OH
This is a mole of Ca₅ (PO₄)₃OH
In this compound we have:
5 moles of Ca
3 mole of P
13 mole of O
1 mole of H
So,
In 1 mole of Ca₅ (PO₄)₃OH, we have 13 moles of oxygen
the value of δh° for the reaction below is -72 kj. ________ kj of heat are released when 80.9 grams of hbr is formed in this reaction. h 2 (g) br 2 (g) → 2hbr (g)
Answers
Answer:
Explanation:
To determine the heat released when a specific amount of a substance is formed in a reaction, we need to use the concept of stoichiometry and the given enthalpy change (ΔH°) for the reaction.
The balanced equation for the reaction is:
H2(g) + Br2(g) → 2HBr(g)
Given:
ΔH° = -72 kJ
Mass of HBr formed = 80.9 grams
To calculate the heat released, we can use the equation:
Heat released = (ΔH° / mol of reaction) * (moles of HBr formed)
First, we need to determine the moles of HBr formed. We can use the molar mass of HBr to convert the given mass to moles:
Molar mass of HBr = 1.01 g/mol + 79.90 g/mol = 80.91 g/mol
moles of HBr formed = mass of HBr formed / molar mass of HBr
= 80.9 g / 80.91 g/mol
= 0.999 mol (approximately 1 mol)
Now, we can calculate the heat released:
Heat released = (-72 kJ / 1 mol) * (1 mol)
= -72 kJ
Therefore, 72 kJ of heat are released when 80.9 grams of HBr is formed in this reaction.
Learn more about heat of reaction: https://brainly.com/question/27092803
#SPJ11
25. 0 L of hydrogen gas at 780 mmHg and 25 C expands to 65. 0L and is subsequently heated to 35 C what’s the new pressure
Answers
The new pressure of the hydrogen gas is approximately 571 mmHg.
To determine the new pressure of the gas, we can use the ideal gas law equation, which states that PV = nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature in Kelvin.
First, we need to convert the given temperatures from Celsius to Kelvin by adding 273.15 to each temperature:
Initial temperature (T1) = 25 + 273.15 = 298.15 K
Final temperature (T2) = 35 + 273.15 = 308.15 K
Next, we can rearrange the ideal gas law equation to solve for the final pressure (P2):
P1V1/T1 = P2V2/T2
Substituting the given values into the equation:
(780 mmHg)(25.0 L)/(298.15 K) = P2(65.0 L)/(308.15 K)
Simplifying the equation, we find:
P2 = (780 mmHg)(25.0 L)(308.15 K) / (298.15 K)(65.0 L)
Calculating this expression will give us the new pressure, which is approximately 571 mmHg.
Learn more about ideal gas law equation here:
https://brainly.com/question/11544185
#SPJ11