What is the electron configuration and lewis structure of { }_{49} In? What is the electron configuration and lewis structure of { }_{49} {In}^{-5} ?
Answers
There are six dots in total. The fifth shell has two dots, and the sixth shell has four dots. The charge of -5 is represented by placing brackets around the symbol and a negative sign outside the brackets.
The element with an atomic number of 49 is indium, with the symbol In. Indium has 49 electrons in its neutral state, and the electron configuration is [Kr]4d105s25p1. 4d10 5s2 5p1 is the abbreviated form of this configuration. The electron configuration and Lewis structure for { }_{49} In are presented below: In: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p1The Lewis structure of In is a simple dot diagram with one dot to represent the one valence electron in its outermost shell.
This is a straightforward electron configuration to learn, and it is one of the most basic. Indium's ion, In-5, has a charge of -5 and has lost five electrons from its neutral state. In its neutral state, indium has three valence electrons; however, when it becomes a negative ion, it gains two more. Indium loses five electrons to form In5-5, which has a noble gas electron configuration of Kr, which is equivalent to the electron configuration of 1s2 2s2 2p6 3s2 3p6.Indium's ion, In-5, has five more electrons than the neutral atom.
It has a total of 54 electrons. When forming the ion, the electrons are first lost from the outermost shell. The electron configuration and Lewis structure for { }_{49} {In}^{-5} are presented below:In5-: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6The Lewis structure for In5- is identical to that of In, but there are now five additional electrons.
To know more about electrons visit:
https://brainly.com/question/12001116
#SPJ11
Related Questions
Calculate the standard reaction enthalpy for the reaction below:
3Fe2O3(s) → 2Fe3O4(s) + ½O2(g)
Answers
The standard reaction enthalpy for the given reaction is +235.8 kJ/mol.
What is the standard reaction enthalpy of reaction?The standard reaction enthalpy (ΔH°) for the given reaction is determined as follows:
Equation of reaction: 3 Fe₂O₃ (s) → 2 Fe₃O₄ (s) + ½ O₂ (g)
The standard enthalpy of formation values for Fe₂O₃ (s), Fe₃O₄(s), and O₂(g) is used to calculate the standard reaction enthalpy.
ΔH° = [2 × ΔH°f(Fe₂O₃)] + [½ × ΔH°f(O₂)] - [3 × ΔH°f(Fe₃O₄)]
where;
ΔH°f(Fe₂O₃) = -824.2 kJ/mol
ΔH°f(Fe₃O₄) = -1118.4 kJ/mol
ΔH°f(O₂) = 0 kJ/mol
ΔH° = [2 × (-1118.4 kJ/mol)] + [½ × 0 kJ/mol] - [3 × (-824.2 kJ/mol)]
ΔH° = -2236.8 kJ/mol + 0 kJ/mol + 2472.6 kJ/mol
ΔH° = 235.8 kJ/mol
Learn more about standard reaction enthalpy at: https://brainly.com/question/15174388
#SPJ1
What is the mass of a piece of iron that releases 179.85 joules of heat as it cools from 51.29 degrees Celsius to 31.42 degrees Celsius? The specific heat of iron is 0.450 J/gC; please answer to two digits after the decimal point.
Answers
Answer:
20.11 g.
Explanation:
What is given?
c (specific heat of iron) = 0.450 J/g °C.
Q (heat energy) = 179.85 J.
ΔT (change of temperature) = |31.42 °C - 51.29 °C| = 19.87 °C.
What do we need? Mass of iron (m)
Step-by-step solution:
Let's see the formula of specific heat:
\(c=\frac{Q}{m\cdot\Delta T}\)Where c is specific heat, Q is heat energy, m is mass and ΔT is the change of temperature.
We just have to solve for 'm' to find the mass of iron and replace the given data that we have, like this:
\(m=\frac{Q}{c\cdot\Delta T}=\frac{179.85\text{ J}}{0.450\text{ }\frac{J}{g\cdot\degree C}\cdot19.87\degree C}=20.11\text{ g.}\)The mass of the iron would be 20.11 g.
Students conduct an experiment:
They placed Vinegar in a bottle and Baking Soda in a ballon...
They then placed the ballon on the bottle and proceeded to drop the baking soda into the bottle.
The students drew the following:
EXPLAIN BELOW THE PICTURE WHAT KIND OF CHANGE DID THEY OBSERVE AND THE PROOF.
Your answer:
Answers
Answer:
The balloon becomes inflated
Explanation:
The equation of the reaction between baking soda (sodium bicarbonate) and vinegar(ethanoic acid) is shown below;
NaHCO3 + HC2CH3O2 ------> NaC2H3O2 + H2O + CO2
The gas (CO2) evolved in the process leads to the inflation of the balloon dropped on the bottle in which the reaction is taking pace.
This observation provides evidence that a gas was really evolved in the reaction.
2 moles of NO, was placed in an empty I dm' bottle and allowed to reach equilibrium according to the equation:
At equilibrium, 1.2 moles of N,O, dissociated. Calculate the value of the equilibrium constant for the reaction at that
temperature.
Answers
2NO(g) ⇌ N2(g) + O2(g)
According to the problem statement, 2 moles of NO were placed in a 1 dm^3 bottle and allowed to reach equilibrium, and at equilibrium, 1.2 moles of NO had dissociated. This means that the initial concentration of NO was:
[NO]initial = 2 mol / 1 dm^3 = 2 M
And the concentration of NO at equilibrium is:
[NO]equilibrium = (2 - 1.2) mol / 1 dm^3 = 0.8 M
Since the stoichiometry of the balanced equation is 2:1:1 for NO, N2, and O2, respectively, the equilibrium concentrations of N2 and O2 will also be 0.6 M.
The equilibrium constant (Kc) can be calculated using the equilibrium concentrations of the reactants and products, raised to the power of their stoichiometric coefficients. Therefore:
Kc = ([N2][O2]) / ([NO]^2)
Substituting the equilibrium concentrations into the equation, we get:
Kc = (0.6 M x 0.6 M) / (0.8 M x 0.8 M)
Kc = 0.5625
Therefore, the value of the equilibrium constant for the reaction at that temperature is 0.5625. Note that the units of Kc depend on the stoichiometry of the balanced equation. Since the stoichiometric coefficients are all 1, the units of Kc in this case are M^-1
what is the difference between an element and a compound wht is the differeence between ionic bonds and covalent bonds
Answers
An element is a pure substance that cannot be broken down into simpler substances by chemical means. It is made up of atoms that have the same number of protons in their nuclei.
Examples of elements include oxygen, carbon, and hydrogen. A compound, on the other hand, is a pure substance made up of two or more elements that are chemically combined in a fixed ratio. Examples of compounds include water (H2O) and carbon dioxide (CO2).
Ionic bonds are formed when two atoms have a large difference in electronegativity, resulting in the transfer of electrons from one atom to another. This results in the formation of positively and negatively charged ions, which are held together by electrostatic attraction. Covalent bonds, on the other hand, are formed when two atoms share one or more pairs of electrons. This sharing of electrons results in the formation of a molecule.
In summary, the key difference between an element and a compound is that an element is a pure substance made up of only one type of atom, while a compound is a pure substance made up of two or more elements that are chemically combined. The difference between ionic and covalent bonds is the way in which electrons are shared or transferred between atoms. Ionic bonds involve the transfer of electrons, while covalent bonds involve the sharing of electrons.
To know more about nuclei visit:
https://brainly.com/question/32368659
#SPJ11
hydrogen fuel can be produced from methane or by electrolysis of water. as of 2020, the majority of hydrogen (∼95%) is produced from fossil fuels by steam reforming or partial oxidation of methane and coal gasification with only a small quantity by other routes such as biomass gasification or electrolysis of water.
Answers
Natural gas contains methane (CH\(4\)), which is used to produce hydrogen by thermal processes like stem-methane reformation and partial oxidation.
In steam-methane reforming, which is the most common method to produce hydrogen, high temperature steam (700°C - 1000°C). The pressure required is 3-25 bar pressure, in the presence of a catalyst. Steam reforming is an endothermic process. Carbon monoxide and steam are reacted using catalyst to produce carbon dioxide and hydrogen.
Partial oxidation is an exothermic process. It is much faster than steam reforming. This method initially produces less hydrogen.
These processes are more preferred because the use of petroleum is low and the emissions is also low. Total greenhouse gases emission is cut.
To learn more about hydrogen fuels ;
https://brainly.com/question/22823816
#SPJ4
5. 4.50 moles of a certain gas occupies a volume of 550.0 mL at 5.000ºC and 1.000 atm. What would the volume be if 10.50 moles are present at 27.00°C and 1.250 atm? HELPPP
Answers
Answer: The final volume is 1108 ml
Explanation:
According to combined gas law:
\(\frac{P_1V_1}{n_1T_1}=\frac{P_2V_2}{n_2T_2}\)
Where :
\(P_1\) = initial pressure = 1.000 atm
\(V_1\) = initial volume = 550.0 ml
\(n_1\)= initial moles = 4.50
\(T_1\) = initial temperature = \(5.000^0C=(273+5.000)=278.00K\)
\(P_2\) = final pressure = 1.250 atm
\(V_2\) = final volume = ?
\(n_2\)= final moles = 10.50
\(T_2\) = final temperature = \(27.00^0C=(273+27.00)K=300.0K\)
\(\frac{1.000\times 550.0}{4.50\times 278.00K}=\frac{1.250\times V_2}{10.50\times 300.0}\)
\(V_2=1108ml\)
Thus final volume is 1108 ml
1)
In C4 (Carbon 4) pants, phosphenolpyruvate (PEP) reacts with carbon dioxide to directly generate following compound in mesophyll cell.
Select one:
a.
ATP
b.
GTP
c.
oxygen
d. oxaloacetate
Answers
In C4 (Carbon 4) pants, phosphenolpyruvate (PEP) reacts with carbon dioxide to directly generate oxaloacetate in mesophyll cell. The correct answer is option d.
What is C4 photosynthesis?C4 photosynthesis is a mechanism for carbon fixation in which carbon dioxide is first fixed into a four-carbon molecule and then transported to specialized cells (bundle sheath cells) in plant leaves. The pathway was discovered in 1967 in the family Amaranthaceae (Chenopodiaceae), but it is now known to exist in at least 19 plant families, including many important crop species such as maize, sorghum, and sugarcane.
In C4 photosynthesis, phosphenolpyruvate (PEP) reacts with carbon dioxide to produce oxaloacetate in mesophyll cell. The mesophyll cell is a kind of photosynthetic cell in the leaves of vascular plants that are responsible for photosynthesis.
The compound is then converted to malate or aspartate and transported to the bundle sheath cell, where carbon dioxide is released and fixed by the enzyme Rubisco (ribulose-1,5-bisphosphate carboxylase/oxygenase). Therefore, the correct answer is option D.
To know more about C4 photosynthesis refer to-
brainly.com/question/14670279#
#SPJ11
A substance is said to hygroscopic if it
absorbs
(a) Carbon (IV) oxide from the atmosphere
(b) from the surrounding
(c) Moisture from the atmosphere to form
a solution
(d) Moisture from the atmosphere without
dissolving in it.
Answers
I'm very confused on Solubility and Temperature. Please Help!
Imagine you have two beakers. Both beakers are filled with the same amount of water. The water in both beakers is the same temperature as well. You add 50 g of Substance A to the first beaker, and 50 g of Substance B to the second beaker. After stirring both beakers, there is a small pile of Substance B at the bottom of the second beaker. None of Substance A is visible in the first beaker. Which of the following statements is true?
Select one:
a. Substance B is not soluble in water
b. Substance A is not soluble in water
c. Substance A is less soluble in water than Substance B.
d. Substance A is more soluble in water than Substance B.
Answers
When 50 cm³ of hydrocarbon Y is burnt, it reacts with exactly 300 cm³ of oxygen to form 200 cm³ of carbon dioxide. Water is also formed in the reaction. Deduce the equation for this reaction. Explain your reasoning.
Answers
Answer:
C2H4 + 3O2---> 2CO2 + 2H2O
Explanation:
The volume of the gas is directly proportional to the no. of moles of the gas at constant temperature and pressure.
So Volume of O2 : Volume of CO2 = 300: 200
= 3:2
So we can add 3 and 2 stoichiometric coefficients to O2 and CO2 respectively.
Then we can see that out of total 3×2 atoms of O, 2×2 atoms of O have been incorporated into CO2. Therefore the remaining 2 O should be in the H2O ( during complete combustion of hydrocarbon we get CO2 and H2O as products)
So we can add 2 in front of H2O..
Then calculate the total no. of C and H atoms in the products side to find the no. of C and H atoms in the hydrocarbon.
C=2
H=4
So the hydrocarbon burnt has the formula C2H4.
To solve such this we must know the concept of combustion reaction. Therefore, The balanced chemical reaction will be
C\(_2\)H\(_4\)+ 3O\(_2\)\(\rightarrow\) 2CO\(_2\) + 2H\(_2\)O
What is chemical reaction?Chemical reaction is a process in which two or more than two molecules collide in right orientation and energy to form a new chemical compound. The mass of the overall reaction should be conserved. There are so many types of chemical reaction reaction like combination reaction, double displacement reaction.
Combustion reaction is a reaction in which a substance, basically hydrocarbons burns in presence of oxygen to produce carbon dioxide and water.
At constant temperature and pressure, the number of moles of the gas directly relates to the volume of the gas.
Volume of O\(_2\): Volume of CO\(_2\)
= 300: 200
= 3:2
The chemical reaction will be
C\(_2\)H\(_4\)+ 3O\(_2\)\(\rightarrow\) 2CO\(_2\) + 2H\(_2\)O
Therefore, the chemical reaction will be
C\(_2\)H\(_4\)+ 3O\(_2\)\(\rightarrow\) 2CO\(_2\) + 2H\(_2\)O
Learn more about the chemical reactions, here:
https://brainly.com/question/3461108
#SPJ2
21(3d − 4) + 100 = 58 State the solution. (If all real numbers
are solutions, enter REALS. If there is no solution, enter NO
SOLUTION.)
Answers
The solution to the equation and value of variable 21(3d - 4) + 100 = 58 is d = 2/3.
Solve the equation 21(3d - 4) + 100 = 58, we can begin by simplifying and isolating the variable:
21(3d - 4) + 100 = 58
Distribute 21 to the terms inside the parentheses:
63d - 84 + 100 = 58
Combine like terms:
63d + 16 = 58
Subtract 16 from both sides:
63d = 42
Divide both sides by 63:
d = 42/63
Simplifying the fraction gives:
d = 2/3
The solution to the equation is d = 2/3.
The solution to the equation 21(3d - 4) + 100 = 58 is d = 2/3. By simplifying the equation, we find that dividing both sides by 63 results in the solution of d = 2/3, which satisfies the original equation.
To know more about variable refer here
https://brainly.com/question/15078630#
#SPJ11
true or false, hydrolysis reactions are catabolic reactions that use water to split the reactant into smaller subunits
Answers
False. Hydrolysis reactions are anabolic reactions that use water to split larger molecules into smaller subunits.
Hydrolysis reactions involve the addition of water molecules to break down complex molecules into simpler components. These reactions are anabolic because they require energy to build larger molecules from smaller subunits. The process involves the breaking of chemical bonds through the addition of water molecules. Water molecules are split, and their constituent hydrogen and hydroxyl ions are incorporated into the reactant molecule, resulting in the formation of two or more products.
An example of a hydrolysis reaction is the breakdown of disaccharides, such as sucrose, into monosaccharides like glucose and fructose. In this reaction, a water molecule is added to the glycosidic bond, resulting in the separation of the disaccharide into its individual sugar units. Hydrolysis reactions play a crucial role in digestion, as enzymes break down complex molecules, such as proteins and carbohydrates, into their building blocks for absorption and utilization by the body.
To know more about hydrolysis reaction, visit:
brainly.com/question/30457911
#SPJ11
precipitation reactions wulrsiee rite chemical, complete ionic, and net ionic equations for the following reactions oduce precipitates. use nr to indicate that no reaction occurs. aqueous solutions of potassium iodide and silver nitrate are mixed. aqueous solutions of ammonium phosphate and sodium sulfate are mixed.
Answers
The chemical reaction involves when aqueous solution of potassium iodide and silver nitrate are mixed is,
KI (aq.) + AgNO3 (aq.) ----> KNO3 (aq.) + Ag I (s)
Net ionic equation,
Ag+(aq.) + I- (aq.) ----> Ag I (s)
complete Ionic equation,
K+ + I- + Ag+ + NO3- ----> K+ + NO3- + Ag I (s)
The Precipitation reaction is the reaction in which dissolved substances react to form one or more solid products. Many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution. This reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. The ability to predict these reactions allows scientists to determine which ions are present in a solution and allows industries to form chemicals by extracting components from these reactions.
When aqueous solutions of ammonium phosphate and sodium sulfate are mixed. No reaction forms.
To learn more about Precipitation Reaction please visit:
https://brainly.com/question/28182226?source=archive
#SPJ4
Types of chemical reactions !!!!!

Answers
Answer:
decomposition is the correct answer
Identify the following examples as either physical or chemical change.
(Ill give brainliest if right)

Answers
Answer:
7. Chemical
8. Chemical
9. Physical
10. Physical
11. Physical
12. Physical
Explanation:
If only 0.500 mol of NO2(g) is placed in a 1.0 L container and the reaction is allowed to come to equilibrium, 0.186 mol of N2O4(g) is formed. Find the value of Keq.
Solve using ICE table.
Answers
The equilibrium constant of the system is 11.3.
What is the value of Keq?We know that the equilibrium constant shows the extent to which reactants are converted into products.
Now;
2NO2 ⇄ N2O4
[NO2] = 0.500 mol /1 L = 0.500 M
[N2O4] = 0.186 mol /1 L = 0.186 M
The ICE table is;
2NO2 ⇄ N2O4
I 0.5 0
C -2x +x
E 0.5 - 2x 0.186
The concentration of NO2 at equilibrium = 0.5 - 2(0.186) = 0.128
Keq = 0.186/( 0.128)^2
Keq = 11.3
Learn more about equilibrium constant:https://brainly.com/question/10038290
#SPJ1
Is copper from periodic table matter or not.
Answers
Answer:Copper is a chemical element with symbol Cu and atomic number 29. Classified as a transition metal, Copper is a solid at room temperature.So copper is a pure substance in any form (only copper atoms). ... A mixture of different kinds of metal atoms is called an "alloy" if they are mixed on an atomic scale.
What is the molality of a solution with 6. 5 moles of salt dissolved in 10. 0 kg of water?
Answers
The molality of the solution is 0.65 mol/kg. Molality is defined as the number of moles of solute per kilogram of solvent.
The molality of a solution with 6.5 moles of salt dissolved in 10.0 kg of water can be calculated as follows:
Step 1: Calculate the mass of water in kilograms.
Mass = Density x Volume
Density of water = 1.00 g/cm³
Volume of water = 10.0 L = 10,000 mL = 10,000 cm³
Mass of water = Density x Volume
= 1.00 g/cm³ x 10,000 cm³
= 10,000 g
= 10.0 kg
Step 2: Calculate the molality of the solution.
Molality = moles of solute / mass of solvent (in kg)
We are given moles of solute = 6.5 mol
Mass of solvent = 10.0 kgMolality
= 6.5 mol / 10.0 kg
= 0.65 mol/kg
To learn more about molality, refer:-
https://brainly.com/question/30909953
#SPJ11
An alkyne with six carbon atoms per
molecule has relative molecular mass
of [C = 12, H = 1]
72,
82
84
86
Answers
Explanation:
C6H10
12(6) + 1(10)
= 72 + 10 = 82
The relative molecular mass of the alkyne with 6 carbon atoms is 82 gram/mole.
What is molecular mass?Molecular mass of a compound or a molecule is defined as the mass of the elements which are present in it.The molecular mass is considered to be a bulk quantity not a molecular quantity. It is often an average of the of the masses at many instances.
The molar mass and formula mass are used as synonym for the molar mass.It does not depend on the amount of substance which is present in the sample.It has units of gram/mole.
Molecular masses of an element are given as relative atomic masses while that of compounds is the sum of relative atomic masses which are present in the compound.Relative molecular masses are also calculated by the same method.
In case of an alkyne with formula C₆H₁₀ relative molecular mass is calculated as, 12×6 +1×10=82 g/mole.
Hence, the relative molecular mass of an alkyne with 6 carbon atoms is 82 g/mole.
Learn more about molecular mass,here:
https://brainly.com/question/18446366
#SPJ2
write a balanced chemical equation for the standard formation reaction of gaseous water h2o
Answers
The balanced chemical equation for the standard formation reaction of gaseous water (H2O) is:
2 H2(g) + O2(g) → 2 H2O(g)
In this equation, two molecules of diatomic hydrogen gas (H2) react with one molecule of diatomic oxygen gas (O2) to produce two molecules of gaseous water (H2O).
The coefficients in front of the chemical formulas indicate the stoichiometric ratios of the reactants and products.
To balance the equation, we need to ensure that the number of atoms of each element is the same on both sides. In this case, we have two hydrogen atoms on the left side and four hydrogen atoms on the right.
So we balance the hydrogen by placing a coefficient of 2 in front of H2O. This gives us four hydrogen atoms on each side.
Next, we have two oxygen atoms on the left side and four oxygen atoms on the right side, so we balance the oxygen by placing a coefficient of 2 in front of O2. This results in four oxygen atoms on each side.
By following these steps, we obtain the balanced chemical equation: 2 H2(g) + O2(g) → 2 H2O(g) for the standard formation reaction of gaseous water.
To know more about standard formation reaction, visit:
https://brainly.com/question/13096727
#SPJ11
Predict the missing component of predict the missing component of each reaction.
? + 2NaBr + 2NaCl + Br2
CH,+202 → ?
4
CHO
OHCI
O Cl2
Na
o C2H2 + CO2
O CO2 + 2H20
O C + 2H20
C
O HBr
Answers
Answer:
B) Cl2
C) CO2 + 2H2O
Explanation:
sorry for the late answer
List the four rules radiation has within Earth's atmosphere.
Answers
Answer:
This article throws light upon the four main laws of radiation. The laws are: 1. Kirchoff's Law 2. Stefan-Boltzman's Law 3. Planck's Law 4. ...
Frequency (f):
Time period (τ):
Wave number:
Speed of light (C):
Wave amplitude (A):
Energy, Wavelength and Temperature:
It is given by the following equation:
Trans fatty acids have physical properties like those ofA) w-3 fatty acids. B) cis-fatty acids. C) unsaturated fatty acids. D) saturated fatty acids
Answers
Trans fatty acids have physical properties like those of saturated fatty acids. Both saturated and trans fatty acids are known to be solid at room temperature due to their molecular structure Option D .
Saturated fatty acids have all of their carbon atoms bonded to hydrogen atoms, while trans fatty acids have their carbon-carbon double bonds in a trans configuration rather than a cis configuration. This trans configuration results in a more linear structure that can pack closely together, giving them similar physical properties to saturated fatty acids. This is in contrast to unsaturated fatty acids, which have one or more carbon-carbon double bonds in a cis configuration, leading to a kink in the molecule that prevents them from packing tightly and results in a liquid state at room temperature.
Learn more about Trans fatty acids
https://brainly.com/question/13528495
#SPJ4
Explain how fossil evidence is evidence of plate tectonics.
Answers
Answer:
Answer
Explanation:
Evidence from fossils, glaciers, and complementary coastlines helps reveal how the plates once fit together. Fossils tell us when and where plants and animals once existed. ... This distribution of fossils led to theories that the southern continents were once joined in a supercontinent called Gondwana.
Answer: Evidence from fossils, glaciers, and complementary coastlines helps reveal how the plates once fit together. Fossils tell us when and where plants and animals once existed.
Explanation: I hoped that helped plzz mark as brainliest !!
If amino acids could be produced by random chemical processes: They would probably be all L stereoisomers. They would probably be all D stereoisomers. They would probably be equal L and D stereoisomers
Answers
Answer:
They would probably be equal L and D stereoisomers
Explanation:
All amino acid (except glycine) can occur in two isomeric forms called L- and D- forms, because of the possibility of forming two different enantiomers around the central carbon atom known as the chiral center. The two enantiomers, have identical physical and chemical properties, but their interactions with other chiral molecules may vary.
A carbon chiral center occcurs when the carbon is bonded to four different substituents. Glycine, has no enantiomers because it has two hydrogen atoms attached to the central carbon atom.
When a compounds with chiral centers are synthesized in the laboratory randomly (in the absence of a directing template) left and right-handed molecules ( corresponding to L- and D- forms) of a compound will form in equal amounts known as a racemic mixture. This was the case when Louis Pasteur in 1848, investigated the crystalline sediment that accumulated in wine casks called paratartaric acid or racemic acid, a form of tartaric acid.
1. You are given the number of moles of carbon and must convert it to an equivalent mass using the molar mass from the periodic table. The carbon sample is 0.045 moles.
2. How many moles of potassium are in 525.0 g of pure potassium? Explain
Answers
0.54g is the mass of carbon in 0.045 moles of carbon. Elementary particles shared the same quantity of matter.
What is mass?A body's mass is an inherent attribute. Until the discoveries of the atom as well as particle physics, it was thought to be tied to the amount of matter inside a physical body. It was discovered that various atoms and elementary particles shared the same quantity of matter.
mole = given mass/ molar mass
substituting all the given values in the above equation, we get
0.045 moles = mass/ 12
mass =0.045×12= 0.54g
Therefore, 0.54g is the mass of carbon in 0.045 moles of carbon.
To learn more about mass, here:
https://brainly.com/question/15368078
#SPJ1
1.) Describe the two diagrams of a bottled carbonated beverage shown below as greater
pressure or lower pressure, and then as greater solubility or lower solubility. How do these two
examples illustrate the relationship between the solubility of a gas and its vapor pressure? Explain
2.) On the diagrams below, assume that each beaker contains an equal number of moles
of solute. Label each solution as concentrated or dilute. Then indicate the approximate
relative volumes of each solution by describing the surface level on each beaker.
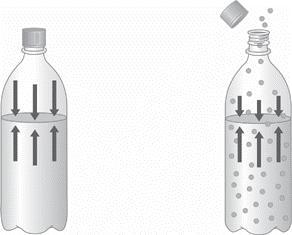

Answers
Answer:
SEE EXPLANATION
Explanation:
If i refer to the bottle at the left hand side as A and the bottle at the right hand side as B. I can say that the bottle A contains solutes which are more soluble than the solutes in bottle B. consequently, A has a lower vapour pressure than B.
We can also see that the pressure in A is much higher than in B(as indicated by the length of the arrows). The higher the pressure, the greater the solubility of the gas. Henry's Law states that: The solubility of a gas in a liquid is directly proportional to the pressure of that gas above the surface of the solution.
Again let me call the beaker on the left A and the beaker on the right B. We can see that the beaker on the left contains 10 ml of solution while the beaker on the right contains 50 ml of solution. It follows that beaker A contains a more concentrated solution since that much amount of solute is contained in a smaller volume than in beaker B as shown in the image.
a sample of CaSO^4 contains 5.2x10^25 molecules. Find the mass of the sample
Answers
1 mole = 6.022 x 10^23 molecules
Number of moles = (5.2 x 10^25 molecules) / (6.022 x 10^23 molecules/mole)
Number of moles ≈ 86.38 moles
Next, we can use the molar mass of CaSO4 to convert moles to grams:
Molar mass of CaSO4 = (1 x 40.08 g/mol) + (1 x 32.06 g/mol) + (4 x 16.00 g/mol)
Molar mass of CaSO4 ≈ 136.14 g/mol
Mass of sample = (86.38 moles) x (136.14 g/mol)
Mass of sample ≈ 11,738.73 g or 11.74 kg (rounded to two decimal places)
Therefore, the mass of the sample is approximately 11.74 kg.
what do the subscripts mean in a molecular formula ?
Answers
Answer:
A molecular formula tells us what atoms and how many of each type of atom are present in a molecule.
Explanation:
If only one atom of a specific type is present, no subscript is used.
For atoms that have two or more present, a subscript is written after the symbol for that atom.
Molecular formulas do not indicate how the atoms are arranged in the molecule.
- Hope this helps! -blazetoxic123
In a molecular formula, the subscripts represent the number of atoms of each element present in a molecule.
The molecular formula gives you the specific combination and quantity of atoms that make up a single molecule of a compound.
For example, consider the molecular formula of water, which is H₂O:
"H" represents hydrogen.
"O" represents oxygen.
The subscript "2" next to "H" indicates that there are two hydrogen atoms in one molecule of water.
The absence of a subscript next to "O" means that there is only one oxygen atom in one molecule of water.
So, the molecular formula H₂O tells us that a water molecule is composed of two hydrogen atoms and one oxygen atom bonded together.
Here are a few more examples to illustrate the importance of subscripts in molecular formulas:
Methane: CH₄ (One carbon atom and four hydrogen atoms)
Carbon dioxide: CO₂ (One carbon atom and two oxygen atoms)
Ethanol: C₂H₅OH (Two carbon atoms, six hydrogen atoms, and one oxygen atom)
The subscripts are crucial in distinguishing between different compounds with the same elements. For instance, carbon monoxide (CO) and carbon dioxide (CO₂) have different molecular structures and properties, even though they both contain carbon and oxygen. The subscripts in the molecular formula help to specify the unique arrangement of atoms within each molecule.
Learn more about molecular formula from the link given below.
https://brainly.com/question/34012285
#SPJ6