Answers
Answer:
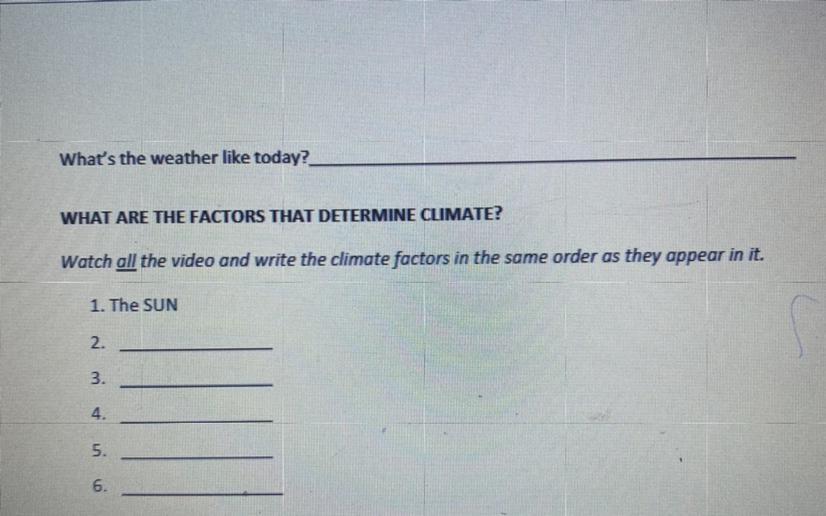
Latitude
ocean currents
Wind
Elevation
Water
Related Questions
Explain why different liquids do not reach the same height in capillary tubes of the same diameter. Please choose the best explanation. a) Different liquids have different intermolecular forces. b) The stronger the intermolecular forces within the liquid, the higher the liquid rise in the capillary tube. c) Cohesive forces stronger than adhesive forces reduce the height of the liquid in the capillary tube, whereas adhesive forces stronger than cohesive forces increase the height of the liquid. d) Polar molecules climb higher than nonpolar molecules. e) The larger the dipole moment, the higher the liquid rises. f) The height of the liquids in the capillary tubes depends on the density of the liquid.
Answers
Answer:
c) Cohesive forces stronger than adhesive forces reduce the height of the liquid in the capillary tube, whereas adhesive forces stronger than cohesive forces increase the height of the liquid.
Explanation:
Two types of forces bring about capillary action.
One is cohesion, which is the intermolecular attraction between like molecules (that is, the liquid molecules). The second force, called adhesion, is an attraction between unlike molecules, such as those in a liquid and in the sides of a glass tube.
If adhesion is stronger than cohesion, the contents of the tube will be pulled upward. This process continues until the adhesive force is balanced by the weight of the liquid in the tube.

the pressure on 20 milliliters of a gas at constant temperature is changed from 4 atmospheres to 2 atmospheres. what is the new volume of the gas?
Answers
The new volume of the gas whose pressure was changed would be = 40 milliliters.
How to calculate the new volume of the given gas?The initial volume(V1)of the gas= 20ml
The initial pressure(P1) = 4 atm
The final pressure(P2) = 2 atm
The final volume(V2) = ?
Using the general gas formula;
P1V1 = P2V2
V2 = P1V1/P2
= 4×20/2
= 40ml
Learn more about volume here:
https://brainly.com/question/27710307
#SPJ1
At 25 oC , the following liquids have density:
water - 0.9982 g/mL; toluene - 0.8669 g/mL; chloroform - 1.4832 g/mL
What mass would a 10.00-mL sample of each of the liquids?
Answers
Answer:
Water- 9.982 grams
toluene-8.669 grams
chloroform - 14.832 grams
Explanation:
the units of density are listed as g/mL. So for every mL of substance you have, you will have that many grams.
Water- .9982g/mL * 10 mL
As you can see, the units of "mL" cancel each other out, leaving just the gram amount of water.
evidence and reasning on when puppies are going to be born
Answers
Answer:
Keep a close eye on her in case she starts giving birth to the next pup at the same time. A greenish/brown discharge may suggest a placenta has separated. If you see this, a puppy should be born within the next 2-4 hours.
If your equation includes 7(CrO4)2, how many Cr's are there?
If your equation includes 7(CrO4)2, how many O's are there?
Answers
If an equation includes 7(CrO₄)₂, the numbers of Cr's and O's atoms that are there are 14 and 56 respectively.
How to calculate number of atoms?The number of atoms present in a chemical compound can be calculated by multiplying the subscript of the particular element by any coefficient.
According to this question, 7 moles of chromate with the chemical formula; (CrO₄)₂ is given. The number of oxygen and chromium atoms in this compound can be calculated as follows:
Chromium = 7 (coefficient) × 2 = 14 atomsOxygen = 7 (coefficient) × 8 = 56 atomsLearn more about no of atoms at: https://brainly.com/question/14190064
#SPJ1
which of the following statements about adp/atp is true g following hydrolosis both atp and adp can release one inorganic phosphate
Answers
Both ATP and ADP can release one inorganic phosphate. The given statement about ATP/ADP is true.
Adenosine Tri Phosphate (ATP) and Adenosine Di Phosphate (ADP) are two critical organic compounds in the metabolism and are essential to the flow of energy in living cells. Hydrolysis is a specific type of chemical reaction in which water molecules break one or more than one chemical bonds.
Hydrolysis of ATP is an exorganic reaction in which one molecule of ATP reacts with one molecule of water to generate one molecule of ADP and One molecule of inorganic phosphate along with some energy.
Hydrolysis of ADP is also an exorganic reaction in which one molecule of ADP reacts with one molecule of water to generate one molecule of Adenosine Mono Phosphate (AMP) and One molecule of inorganic phosphate along with some energy.
Hence, the hydrolysis of both ATP and ADP leads to the generation of inorganic phosphate. Therefore, the given statement is true.
Learn more about ATP: https://brainly.com/question/24664390
#SPJ4
Using the periodic table, choose the more reactive nonmetal.
Br or As
Answers
First of all, As is a metalloid, not a nonmetal. Second of all, as the rows get higher on the periodic table, the elements are more reactive. As you move right on the periodic table, the elements become more reactive.
a pharmaceutical company wants to extract an ingredient from pomace supplied by a certain food processing plant. which process is the most efficient with the lowest environmental impact
A. Catalyst extraction
B. High pressure, high temperature water extraction
C. Organic solvent extraction
D. Enzyme extraction
Answers
Answer: high pressure, high temperature water extraction
Explanation:
Answer:
C). high pressure, high temperature water extraction
Explanation:
Iron and Chlorine gas react according to the following balanced equation: 2 Fe(S) + 3 Cl2 (g) 2 FeCl3(s) a) Calculate the molar mass in grams of “one mole” of each of the following: Fe ________ Cl2 __________________ FeCl3 ______________
Answers
The molar mass in grams of "one mole" of each substance is:
Fe: 55.845 g/mol
\(Cl_2\): 70.906 g/mol
\(FeCl_3\): 162.204 g/mol
To calculate the molar mass in grams of "one mole" of each substance, we need to determine the atomic masses of the elements involved in the equation.
The atomic mass of iron (Fe) is 55.845 g/mol.
For chlorine (\(Cl_2\)), we need to consider that the molar mass of \(Cl_2\) is twice the atomic mass of chlorine because the formula shows that two chlorine atoms combine to form one molecule of \(Cl_2\). The atomic mass of chlorine is 35.453 g/mol, so the molar mass of \(Cl_2\) is 2 * 35.453 g/mol = 70.906 g/mol.
The formula for iron(III) chloride (\(FeCl_3\)) indicates that one mole of \(FeCl_3\)contains one mole of iron and three moles of chlorine. Therefore, we can calculate the molar mass of \(FeCl_3\)by summing the atomic masses of iron and chlorine:
Molar mass of \(FeCl_3\)= (1 * atomic mass of Fe) + (3 * atomic mass of Cl)
Substituting the values, we have:
Molar mass of \(FeCl_3\) = (1 * 55.845 g/mol) + (3 * 35.453 g/mol)
= 55.845 g/mol + 106.359 g/mol
= 162.204 g/mol
For more such questions on molar mass visit:
https://brainly.com/question/837939
#SPJ8
A given solution has 42.5 g NaNO3 in 1500 mL of water. What is the molarity of this solution? Show your work, rounding the atomic mass of each element to the nearest tenth and your final answer to the nearest hundredth
Answers
Explanation:
here's the answer to your question

The Tollen's test is the reaction of aldehydes with silver(l) ions in basic solution to form silver metal and a carboxylate. 2 Ag+ + + 3 OH- - HR 2 Ag +_ W + 2 H2O ÃR Which species is being oxidized in the reaction? Choose... Which species is being reduced in the reaction? Choose... Which species is the visual indicator of a positive test? v Choose... Carboxylate ion Aldehyde Silver metal Water Silver(1) ion Hydroxide ion
Answers
Answer:
Explanation:
When Tollen's test is done by aldehyde , silver ion is converted into silver which forms a layer which looks like a mirror.
Ag⁺ + e = Ag
It is a reduction process where silver(1) ion is reduced to metallic silver.
Aldehyde is oxidized to carboxylate ion.
CH₃CHO + 2 OH⁻ = CH₃COO⁻ + H₂O + H⁺ + 2e
Visual indicator is silver metal which forms silver mirror at the bottom of test tube .
what equipment do you need to conduct the flame tests?
Answers
Answer:
you use hairspray and then use the lighter flame touching the hairspray as its spraying out and there is a way to make a sort of blow torch type of thing I recommend not doing so because its dangerous
Which of the following is the correct equation for the reaction below?
A. CH (g) + O2 (g) CO (g) + H2O (g)
B. CH (g) + 2O (g) CO (g) + 2HO (g)
C. CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (g)
D. CH4 (g) + 2O2 (g) CO2 (g) + H2O (g)
Answers
Answer:
(c) is the correct answer
CH4(g)+2O2(g)⟶CO2(g)+2H2O(l)
please mark me as brainlist
How many moles of CH2 must react in order to produce 50 moles
Answers
Q1. Consider the gravitational interactions among Earth, the Sun, and the Moon. Does this constitute a system? If so, what are its boundaries? Is it open or closed? What forms of energy are involved?
Answers
When two objects descend towards one another, the potential energy related to the gravitational field is released (transformed into kinetic energy).
The potential energy that a huge item has in relation to a different massive object due to gravity is known as gravitational energy and gravitational potential energy. When two objects descend towards one another, the potential energy related to the gravitational field is released (transformed into kinetic energy). Bringing two things farther apart increases the gravitational potential energy.
To know more about gravitational, here:
https://brainly.com/question/3009841
#SPJ1
. Write the two resonance hybrids for the carbocation that would be formed by protonation at C-1 of 2-methyl-1,3-pentadiene. Without doing a calculation, would you expect C-2 or C-4 (the two end carbons of the allylic cation) to have the most positive charge on it
Answers
Answer:
Answer is explained in the explanation section below.
Explanation:
This question is requires the diagram, which I have drawn and it is attached in the attachment below. Please for this answer, refer to the diagram attached in the attachment below.
Referring to the diagram, attached. As we know that, Allylic secondary carbocations are more stable than Allylic tertiary carbocations.
Hence,
C2 will have a more positive charge since a tertiary carbocation (C2) is more stable than a secondary carbocation (C4). Therefore, the resonance structure will favor the positive charge at C2.

The resonance structure formed for 2-methyl 1-3 penatdiene are allylic secondary and tertiary carbocation compounds.
What is protonation?Protonation is the addition of hydrogen or the protons to the carbon in an organic compound. The addition of hydrogen takes place at the carbon that forms the stable compound.
The protonation of 2-methyl-1,3 pentadiene is given in the image attached.
The expected carbocation in the structure with the positive charge is C-2, as it forms a more stable product than C-4 due to less repulsion.
Learn more about protonation, here:
https://brainly.com/question/1013246

The N2O decomposition, 2 N2O (g) → 2 N2 (g) + O2 (g), is a zero order reaction that has a reaction rate constant of 2.00 × 10−3 M/s. Initially, 2.00 moles of N2O gas are infused into a 1.00 L vessel at 300. K.Calculate the pressure inside the vessel at 100. seconds after the start of the reaction. Temperature is held constant.
Answers
Answer:
2.00n2g
Explanation:
hope its attachments
You should run an experiment several times to make sure your results are consistent. In the preceding phrase, what does "consistent" mean?
Answers
Answer:
consistent
Explanation:
in this phrase, consistent means that your results do not falter and prove that your answer is performed correctly and efficiently. if an experiments results are repeated and you get a different outcome, you should restart your process and evaluate what mightve hone wrong.
True or false
The sodium atom is a cation
Answers
MARK ME AS BRAINLIST
0.350 moles of H2O is equivalent to (blank amount) molecules of water.
Answers
Answer:
is has around 1/5 lyrics of water
Answer:
\(1 \: mole \: = \: 6.02 \times {10}^{23} \: molecules \\ 0.350 \: moles \: = \: (0.350 \times 6.02 \times {10}^{23} ) \: molecules \\ = 2.107 \times {10}^{23} \: molecules\)
Which statement best illustrates how mixtures and pure substances are different?
Mixtures have color; pure substances are colorless.
Mixtures have various odors; pure substances are odorless.
Mixtures are found on the periodic table; pure substances are not.
Mixtures are physically combined; pure substances are chemically combined.
Answers
Answer:
Mixtures are physically combined and pure substances are chemically combined.A compound is found to contain 3.622 % carbon and 96.38 % bromine by mass. To answer the question, enter the elements in the order presented above.
QUESTION 1: The empirical formula for this compound is .
QUESTION 2: The molecular weight for this compound is 331.6 amu. The molecular formula for this compound is
Answers
Question 1 : the empirical formula for the compound is CBr₄.
Question 2 : the molecular formula for the compound is CBr₄.
To determine the empirical formula and molecular formula of the compound, we need to analyze the given percentage composition and molecular weight. Let's go through the process step by step:
Empirical Formula:
The percentage composition by mass states that the compound contains 3.622% carbon and 96.38% bromine. We can assume a 100g sample of the compound to simplify the calculations.
Mass of carbon = (3.622/100) * 100g = 3.622g
Mass of bromine = (96.38/100) * 100g = 96.38g
Next, we need to find the moles of each element. We can use their atomic masses to convert the masses to moles.
Atomic mass of carbon (C) = 12.01 g/mol
Atomic mass of bromine (Br) = 79.90 g/mol
Moles of carbon = Mass of carbon / Atomic mass of carbon = 3.622g / 12.01 g/mol ≈ 0.3017 mol
Moles of bromine = Mass of bromine / Atomic mass of bromine = 96.38g / 79.90 g/mol ≈ 1.205 mol
To find the simplest whole-number ratio between the elements, we divide both moles by the smallest number of moles (0.3017 mol in this case):
Moles of carbon (C) = 0.3017 mol / 0.3017 mol = 1
Moles of bromine (Br) = 1.205 mol / 0.3017 mol ≈ 4
Therefore, the empirical formula for the compound is CBr₄.
Molecular Formula:
Given that the molecular weight (molar mass) of the compound is 331.6 amu, we need to compare it with the empirical formula weight.
Empirical formula weight = (Atomic mass of carbon × Number of carbon atoms) + (Atomic mass of bromine × Number of bromine atoms)
= (12.01 amu × 1) + (79.90 amu × 4) = 12.01 amu + 319.6 amu = 331.61 amu
The molecular weight is very close to the empirical formula weight, indicating that the empirical formula represents the molecular formula as well. Therefore, the molecular formula for the compound is also CBr₄.
Know more about Molecular formula here:
https://brainly.com/question/15960587
#SPJ8
16) Select the best answer.
Round the answer to the correct number of significant figures.
10.05
2.8899 = 29.043495
29.0435
29.04
29.043
29
Answers
29 is not the best answer depends on the context and the rules for significant figures.
What is best answer?
The best answer depends on the context and the rules for significant figures. If we assume that we need to round to three significant figures:
10.05 has three significant figures, so it is already rounded correctly.2.8899 has four significant figures, so we need to round it to three significant figures. The third significant figure is 9, which is greater than 5, so we round up the second significant figure (which is 8) to 9. Therefore, 2.8899 rounded to three significant figures is 2.89.29.0435 has five significant figures, so we need to round it to three significant figures. The third significant figure is 0, which is less than 5, so we do not round up the second significant figure (which is 4). Therefore, 29.0435 rounded to three significant figures is 29.0.29.04 has four significant figures, so it is already rounded correctly.29.043 has four significant figures, so we need to round it to three significant figures. The third significant figure is 3, which is less than 5, so we do not round up the second significant figure (which is 4). Therefore, 29.043 rounded to three significant figures is 29.0.29 has one significant figure, so it is not rounded correctly to three significant figures.Therefore, 29 is not the best answer.
To know more about significant figures, visit:
https://brainly.com/question/30465808
#SPJ1
What is a precipitate?
a) insoluble ionic compound
b) metallic solid
c) soluble ionic compound
d) gaseous molecular compound
Answers
Which of these solutions are basic at 25 °C ? A:[OH−]=2.53×10−7 M B:[H3O+]=9.99×10−9 M C:[H3O+]=6.31×10−4 M
Answers
Answer: frfg6
Explanation: 655tyyt
In what areas of the periodic table do you find the most highly reactive elements?
Answers
Answer:
The elements toward the bottom left corner of the periodic table are the metals that are the most active in the sense of being the most reactive.
The most highly reactive elements are typically found at the far left (Group 1) and far right (Group 17) of the periodic table.
Highly reactive elements in the periodic tableGroup 1 elements, also known as alkali metals, are located on the far left of the periodic table. They have one electron in their outermost energy level and are highly reactive due to their tendency to lose that electron to achieve a stable electron configuration. This makes them very reactive with water and other substances.
Group 17 elements, known as halogens, are located on the far right of the periodic table. They have seven electrons in their outermost energy level and are highly reactive due to their strong tendency to gain one electron to achieve a stable electron configuration. This makes them reactive with metals and other elements.
More on the periodic table can be found here: https://brainly.com/question/28747247
#SPJ3
how many elements are present in the compound
Answers
go play mine craft use code wild ............................
How many liters of fluorine gas are needed to form 519 L of sulfur hexafluoride gas if the following reaction takes place at 2.00 atm and 273.15 K : S(s)+ 3F 2 (g) SF 6 (g) ?
Answers
According to ideal gas law, 3404.5 liters of fluorine gas are needed to form 519 L of sulfur hexafluoride gas if the following reaction takes place at 2.00 atm and 273.15 K .
What is ideal gas law?The ideal gas law is a equation which is applicable in a hypothetical state of an ideal gas.It is a combination of Boyle's law, Charle's law,Avogadro's law and Gay-Lussac's law . It is given as, PV=nRT where R= gas constant whose value is 8.314.The law has several limitations.
Substitution of values in equation gives, V= 3×8.314×273//2=3404.5 liters.
Thus, 3404.5 liters of fluorine gas are needed to form 519 L of sulfur hexafluoride gas if the following reaction takes place at 2.00 atm and 273.15 K .
Learn more about ideal gas law,here:
https://brainly.com/question/28257995
#SPJ1
glucose is a six carbon sugar. Albumin is a protein with 607 amino acids. the average molecular weight of a single amino acid is 135 g/mol. there is no reason to run these solutes at the 20 MWCO because
Answers
There is no reason to run these solutes at the 20 MWCO because they are both much smaller than the MWCO of the membrane.
The MWCO (molecular weight cut off) is the molecular weight of a solute at which it will be retained by a membrane during a process such as ultrafiltration or dialysis. If a solute has a molecular weight higher than the MWCO of a membrane, it will be retained and not pass through the membrane. If the molecular weight of a solute is lower than the MWCO, it will pass through the membrane.
In this case, glucose has a molecular weight of 180 g/mol (6 carbons x 12 g/mol per carbon + 6 oxygens x 16 g/mol per oxygen) and albumin has a molecular weight of approximately 81,942 g/mol (607 amino acids x 135 g/mol per amino acid). Both of these solutes have molecular weights that are much lower than 20,000 g/mol, which is a typical MWCO for ultrafiltration or dialysis membranes.
They would both easily pass through the membrane and be lost during the process. Instead, a membrane with a much lower MWCO would be needed if we wanted to retain these solutes during a process such as ultrafiltration or dialysis.
Learn more about glucose here:
https://brainly.com/question/2396657
#SPJ1
34.8 g of Na₂O are used to form a solution with a volume of 450.0 mL L. What is the
molarity?
34.89
Answers
Answer:
1.25 M
Explanation:
(Step 1)
Convert grams to moles using the molar mass of Na₂O.
Atomic Mass (Na): 22.990 g/mol
Atomic Mass (O): 15.999 g/mol
Molar Mass (Na₂O): 2(22.990 g/mol) + 15.999 g/mol
Molar Mass (Na₂O): 61.979 g/mol
34.8 g Na₂O 1 mole
---------------------- x --------------------- = 0.561 moles Na₂O
61.979 g
(Step 2)
Convert milliliters to liters.
1,000 mL = 1 L
450.0 mL 1 L
------------------ x ------------------- = 0.4500 L
1,000 mL
(Step 3)
Calculate the molarity using the molarity ratio.
Molarity = moles / volume (L)
Molairty = 0.561 moles / 0.4500 L
Molarity = 1.25 M