What element has the noble gas configuration [Ar]4s23d8
Answers
Answer:
argon
Explanation:
Answer:
Argon
Explanation:
Related Questions
a sample of water, h2o, has a mass of 24.50 g. calculate the number of water molecules in the sample.
Answers
Therefore, the number of water molecules in the given sample is approximately \(8.18 * 10^2^3\) molecules.
What is Avogadro's number?To calculate the number of water molecules in the given sample, we need to use the concept of Avogadro's number and the molecular weight of water.
The molecular weight of water (H2O) is:
H = 1.008 u (atomic mass units)
O = 15.999 u (atomic mass units)
Molecular weight of H2O = (2 x 1.008 u) + 15.999 u = 18.015 u
Using the molecular weight of water, we can calculate the number of moles of water in the sample:
Number of moles = mass / molecular weight
Number of moles = 24.50 g / 18.015 g/mol
Number of moles = 1.359 mol
Now, using Avogadro's number (\(6.022 *10^2^3\) molecules/mol), we can calculate the number of water molecules in the sample:
Number of water molecules = Number of moles x Avogadro's number
Number of water molecules = \(1.359 mol * 6.022 *10^2^3\) molecules/mol
Number of water molecules =\(8.18 * 10^2^3\)molecules
To know more about molecules visit:-
brainly.com/question/21263612
#SPJ1
How many moles of H3PO4 are contained in 150.0 mL of 18.1 M H3PO4?
Answers
Answer:
Number of moles = 2.72 mol
Explanation:
Given data:
Number of moles of H₃PO₄ present = ?
Molarity of solution = 18.1 M
Volume of solution = 150.0 mL (150/1000 = 0.15 L)
Solution:
Molarity is used to describe the concentration of solution. It tells how many moles are dissolve in per litter of solution.
Formula:
Molarity = number of moles of solute / L of solution
By putting values,
18.1 M = number of moles / 0.15 L
Number of miles = 18.1 M × 0.15 L
Number of moles = 2.72 mol
what is the difference between metals and non non metals
...
Answers
Answer:
Metals tend to be hard, metallic-looking solids, with high electrical and thermal conductivity values and high melting and boiling points. Nonmetals tend to be softer, often colorful elements. They may be solids, liquids, or gases.
Answer: Metals are hard and have high melting and high boiling points and high electrical and thermal conductivity values and nonmetals are softer and are substances like solids,liquids,or gases.
Sita collected 8.6 g of soil and dried it well and found out to be 7g. Find the moisture content of soil.
Answers
Moisture is the water present in the soil that is trapped in the particles. The moisture content of the soil with an initial weight of 8.6 gm and dry weight of 7.0 gm will be 0.228.
What is the moisture content?The moisture content is the estimation of the weight by the subtraction of the dry soil mass from the moist soil mass followed by dividing the value by the weight of the dry soil.
Given,
Weight of moist soil = 8.6 gm
Weight of dry soil = 7.0 gm
Substituting values,
(8.6 - 7.0) ÷ 7.0
= 0.228
Therefore, 0.228 is the moisture content of the soil.
Learn more about moisture content here:
https://brainly.com/question/13768807
#SPJ1
how is an object's speed determined?
Answers
Answer:
Divide the distance the object traveled by the time it took to get there.
Explanation:
To calculate the speed on an object, start by determining how far the object has traveled. Next, figure out the amount of time that the object took to cover that distance. Finally, divide the distance the object traveled by the time it took to get there. Don't forget to label the speed with the correct units of measurement.

At a given temperature, 5.42 atm of F2 and 3.87 atm of Br2 are mixed and allowed to come to equilibrium. The equilibrium pressure of BrF is found to be 1.42 atm. Calculate Kp for the reaction at this temperature.
F2(g) + Br2(g) <=> 2 BrF(g). Give answer to 3 decimal places.
Answers
The equilibrium constant for the given reaction at the given temperature is 0.478.
To calculate Kp for the given reaction, we first need to write the balanced equation and the expression for Kp:
F2(g) + Br2(g) <=> 2BrF(g)
Kp = (PBrF)^2 / (PF2 x PBr2)
Here, PBrF, PF2, and PBr2 are the partial pressures of BrF, F2, and Br2, respectively, at equilibrium. We are given the initial partial pressures of F2 and Br2, as well as the equilibrium pressure of BrF.
To determine the equilibrium partial pressures of F2 and Br2, we can use the stoichiometry of the reaction and the ideal gas law.
Let x be the equilibrium concentration of BrF. Then, the equilibrium concentrations of F2 and Br2 will be (5.42 - x) and (3.87 - x), respectively.
Using the ideal gas law, we can write:
PF2 = (5.42 - x) * (RT/V) and PBr2 = (3.87 - x) * (RT/V)
where R is the gas constant, T is the temperature in kelvin, and V is the volume.
At equilibrium, the total pressure is given by:
Ptotal = PF2 + PBr2 + PBrF = 5.42 + 3.87 + 1.42 = 10.71 atm
Substituting the partial pressures into the expression for Kp, we get:
Kp = (1.42)^2 / [(5.42 - x) * (3.87 - x)]
Simplifying and solving for x, we get:
x = 1.92 atm
Substituting x back into the expressions for PF2 and PBr2, we get:
PF2 = 3.50 atm and PBr2 = 1.95 atm
Finally, substituting all the partial pressures into the expression for Kp, we get:
Kp = (1.42)^2 / (3.50 x 1.95) = 0.478
Therefore, the equilibrium constant for the given reaction at the given temperature is 0.478 (to 3 decimal places).
To know more about equilibrium constant visit: https://brainly.com/question/28559466
#SPJ11
Chose all of the correct statements pure vs mixture

Answers
Answer:
I think that a pure substance is a form of matter that has a constant composition and properties that are constant throughout the sample. Mixtures are physical combinations of two or more elements and/or compounds.
Explanation:
Aluminum oxide (Al2O3) and iron (Fe) react according to the following
equation:
_Al2O3 + _Fe → _Fe304 +_AL
Balancing the equation requires inserting the correct coefficients to reflect
that no atoms are gained or lost in the reaction. Which is the correct set of
four coefficients, in order?
A. 3, 9, 3,6
B. 4, 9, 3,8
c. 1,3,1,2
D. 2, 8, 3,6

Answers
The correct set of coefficients, in order, to balance the equation is: 3, 9, 3, 6. Therefore, option (A) is correct.
To balance the chemical equation \(Al_{2}O_{3}\)+ Fe → \(Fe_{3}O_{4}\) + Al, we need to determine the coefficients that ensure the same number of atoms for each element on both sides of the equation.
In the given answer, A. 3, 9, 3, 6, the coefficients indicate that three molecules of aluminum oxide (\(Al_{2}O_{3}\)) and nine atoms of iron (Fe) react to form three molecules of iron(III) oxide (\(Fe_{3}O_{4}\)) and six atoms of aluminum (Al).
By balancing the equation with these coefficients, we ensure that the number of atoms of each element is conserved. This means that no atoms are gained or lost during the reaction, satisfying the principle of conservation of mass.
Balancing equations is crucial for accurately representing chemical reactions, as it ensures that the reactants and products are in the correct stoichiometric ratios and reflects the conservation of atoms.
Learn more about stochiometric coefficients, here:
https://brainly.com/question/30350845
#SPJ2
the diagram of paper chromatography
Answers
I hope that the attachment helps you..

PLEASE HELP
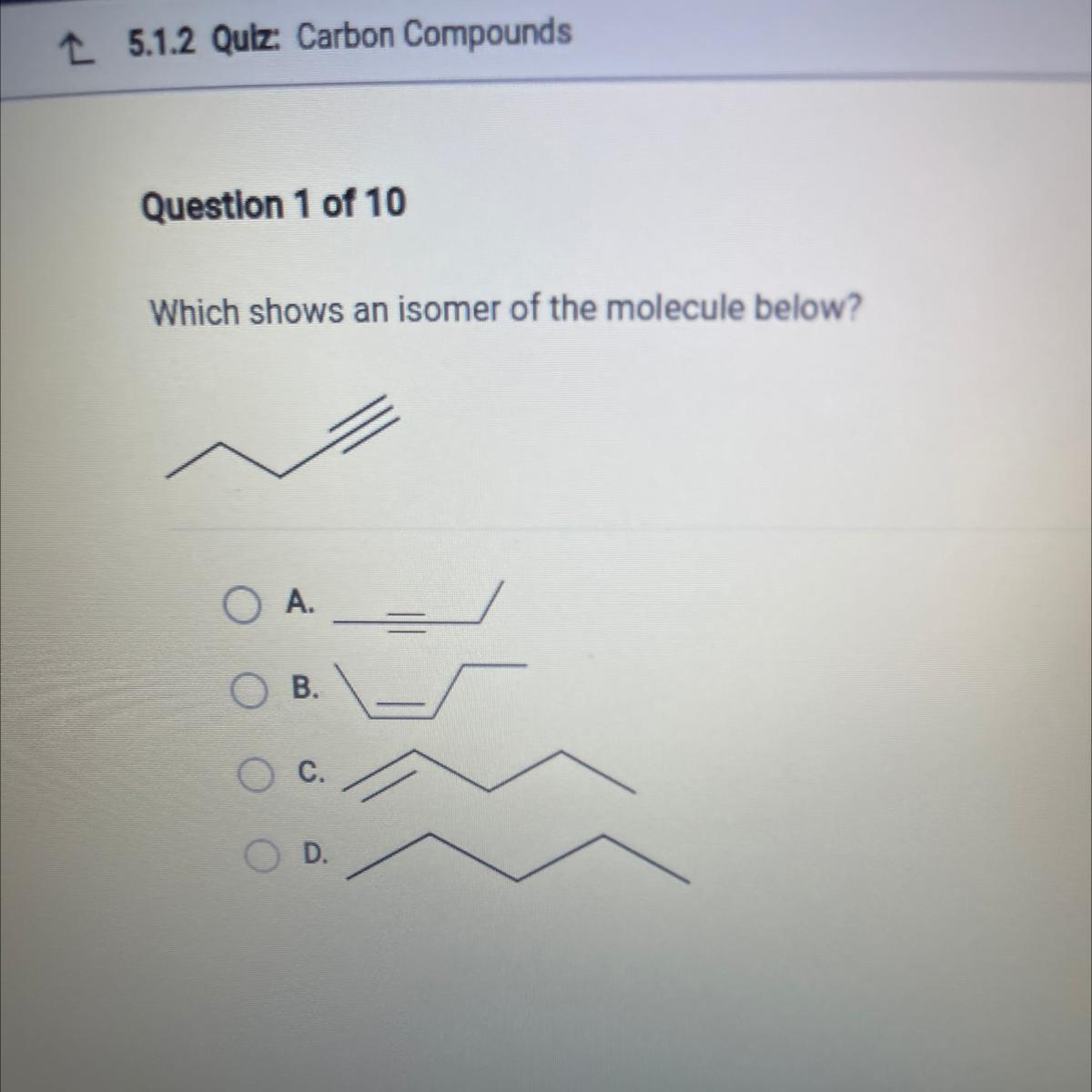
Which shows an isomer of the molecule below?
Answers
Need Help!!
Explain the relationship between volume and temperature (Charles’ Law)
Answers
Answer:
the volume of a give gas simple is directly propotional assolute temperature at constant pressure .the volume of a gavi. amount of gass is inversely propotional ot Its pressure when temperature is help constant
The mass percentage of hydrochloric acid within a solution is 19. 0%. Given that the density of this solution is 1. 095 g/ml, find the molarity of the solution.
Answers
The Molarity of hydrochloric acid is 5.708 M containing mass of 19.0% and the density of the solution is 1.095 g/ml .
Molarity of the solution is defined as the amount of solute in moles divided by the volume of solution in liters.
M = moles of solute/liters of solution.
we can calculate the molarity by dividing the number of moles of solute by the total volume of liters of solution.
let's assume 100g solution. the mass percentage of hydrochloric acid within the solution is 19.0%. and the density of the solution that is give is 1.095g/mole. substituting all these values we can calculate the molarity of the solution.
mass Hydrochloric acid,
100g solution x (19.00g / 100g solution) = 19.00g Hcl
moles of hydrochloric acid is ,
19.00g HCl x (1 mole Hcl / 36.45g HCl) = 0.52126
then the solution is,
100g solution x (1mL solution / 1.095g solution) x (1L / 1000mL) = 0.091324L
molarity HCl = 0.52126 mole / 0.091324L = 5.708M.
To learn more about the Molarity of the solution please visit:
https://brainly.com/question/26528084
#SPJ4
if answered correctly i will give brainlest

Answers
Answer: Earthquake location
Explanation:
Answer:
Volcano chains and arcs, and earthquake locations
A neutral atom contains 12 neutrons and 11 electrons. The number of protons in this atom is
(1) 1 (3) 12
(2) 11 (4) 23
Answers
Answer:
1
Explanation:
fill in the blanks with the words given below-
[Atoms, homogeneous, metals, true, saturated, homogeneous, colloidal, compounds, lustrous]
1.An element which are sonorous are called................
2.An element is made up of only one kind of ....................
3.Alloys are ............................. mixtures.
4.Elements chemically combines in fixed proportion to form ........................
5. Metals are................................... and can be polished.
6. a solution in which no more solute can be dissolved is called a .................... solution.
7. Milk is a .............. solution but vinegar is a .................. solution.
8. A solution is a ................... mixture.
pls help, could not get these answers
Answers
Answer:
1. Metal.
2. Atom.
3. Homogeneous
4. Compounds.
5. Lustrous
6. Saturated.
7. Colloidal; true.
8. Homogeneous.
Explanation:
1. An element which are sonorous are called metal.
2. An element is made up of only one kind of atom.
3. Alloys are homogeneous mixtures.
4. Elements chemically combines in fixed proportion to form compounds.
5. Metals are lustrous and can be polished.
6. A solution in which no more solute can be dissolved is called a saturated solution.
7. Milk is a colloidal solution but vinegar is a true solution.
8. A solution is a homogeneous mixture.
which one is a product of this reaction? ch3ch2mgbr ch3oh ch3ch2-h ch3ch2-och3 ch3ch2-ch3 ch3ch2-oh
Answers
According to the question a product of this reactio is \(CH_3CH_2-OH\) , which is an alcohol.
What is alcohol ?Alcohol is a psychoactive substance that is produced by the fermentation of yeast, sugars, and starches. It can be found in many forms, including beer, wine, and distilled spirits, and is widely consumed around the world. Alcohol has been consumed in some form since ancient times and is commonly used to celebrate special occasions and mark important life events. Consumption of alcohol can be both beneficial and detrimental to health, depending on the amount consumed and the individual's sensitivity. It can have short-term effects on the body such as impaired motor skills and judgement, and long-term effects such as liver damage and increased risk of certain cancers.
This is because the reaction involves the reaction of an alkyl halide (\(CH_3CH_2Br\)) with a magnesium halide (MgBr) to form an alkoxide \((CH_3CH_2-O-MgBr)\). When acid is added, the alkoxide is protonated and the magnesium halide is eliminated, forming an alcohol \((CH_3CH_2-OH)\).
To learn more about alcohol
https://brainly.com/question/29751198
#SPJ4
Give the equation for a supersaturated solution in comparing Q with Ksp.
A. Q ≠ Ksp
B. Q = Ksp
C. Q < Ksp
D. Q > Ksp
E. None of the above.
Answers
Supersaturated solutions are any liquids that contain more solute than is necessary to generate a saturated solution at any given constant temperature. Q > Ksp for a supersaturated solution. The correct option is D.
The ionic product results from ionic species concentrations in either an unsaturated or a saturated solution. The ionic product is referred to as the solubility product when only saturated solutions are taken into account. All forms of solutions can be referred to as ionic products.
The product of the ion concentration that is in equilibrium with the solid substance in a saturated solution is known as the solubility product. Q > Ksp, the solution is supersaturated, and an ionic solid will precipitate.
The correct option is D.
To know more about supersaturated solutions, visit;
https://brainly.com/question/31118122
#SPJ12
Pb(NO3)2 (aq) + 2 NaI (aq) --> PbI2 (s) + 2 NaNO3 (aq)
Starting with with 50.0 grams of Pb(NO3)2 and 30.0 grams of NaI:
A. What is the limiting reagent?
B. How many grams of the excess reactant remains?
C. How many grams of each product is formed?
D. If 12 grams of NaNO3 actually formed in the reaction, what is the percent yield of this reaction?
Answers
Answer:
Explanation:
Moles of Pb(NO3)2 = mass/molecular mass
= 50.0 grams/(207.20*1 + 14.01*2 + 16*6)
= 50.0 grams/331.22
= 0.15 moles
Moles of NaI
= 30/(22.99+126.9)
= 30/149.89
= 0.2 Moles
A. NaI is less 2x Pb(NO3)2 so NaI is the limiting reagent.
B. The ratio is 1 to 2 so there is 0.15 - 0.2/2 = 0.05 mole
or 16.78 grams of Pb(NO3)2 left.
C. As NaI is limiting, only 0.2 Moles of NaNO3 is formed.
Mass = Moles * Molecular Mass
Molecular Mass of NaNO3 can be calculated as:
Na - 22.99
N - 14.01
O - 3(16) = 48
23+14+48 = 85gram / mole
Thus, Mass = 0.2*85 = 17 gram of NaNO3
Mass is conserved in a chemical reaction.
Mass of PbI2 can be calculated as:
50+30-16.78-17
= 46.3 gram of PbI2
Mass =
12.75
Thus, 12.75g of Sodium Nitrate can be formed
Answer:
Explanation:
Pb(NO3)2 (aq) + 2 NaI (aq) --> PbI2 (s) + 2 NaNO3 (aq)
MM for each compound -
Pb(NO3): 207 + 14x2 + 16x3x2 = 331
PI2: 207 + 127x2 = 461
NaI: 23 + 127 = 150
NaNO3: 23 + 14 + 16x3 = 85
Moles of Pb(NO3)2 = 50/331 = 0.15
Moles of NaI = 30/150 = 0.2
Ratio of moles is 1:2
So NaI is limiting
Limited to 0.2/2 = 0.1 mole of Pb(NO3)2
Excess = 0.15 - 0.1 = 0.05 mole
Mass remains = 0.05x331 = 16.55 grams
Moles of NaNO3 formed = Moles of NaI reacted = 0.2
Mass = 0.2x85 = 17 grams
Moles of PbI2 formed = Moles of Pb(NO3)2 reacted = 0.1
Mass = 0.1x461 = 46.1 grams
If 12 grams of NaNO3 actually formed in the reaction,
percent yield = 12/17x100% = 70.6%
What mass of coppe is deposited on the cathode of an electrolytic cell if a current of 2.00 a is passed through a solution of cuso4 for a period of 20.0 minutes?
Answers
Approximately 0.156 grams of copper is deposited on the cathode of the electrolytic cell.
To calculate the mass of copper deposited on the cathode of an electrolytic cell, we can use Faraday's laws of electrolysis and the equation:
The equation is:
M = (Q * Mw) / (n * F)
Where:
M is the mass of the substance deposited (in grams),
Q is the total electric charge passed through the cell (in coulombs),
Mw is the molar mass of the substance (in grams per mole),
n is the number of moles of electrons transferred in the reaction, and
F is the Faraday constant (approximately 96,485 C/mol).
In this case, copper is being deposited on the cathode, and the balanced chemical equation for the electrolysis of CuSO₄ is:
Cu²+ + 2e⁻ → Cu
The number of moles of electrons transferred (n) in this reaction is 2, as indicated by the balanced equation.
Given:
Current (I) = 2.00 A
Time (t) = 20.0 minutes = 20.0 * 60 seconds
= 1200 seconds
First, we need to calculate the total electric charge (Q) passed through the cell using the formula:
Q = I * t
Q = 2.00 A * 1200 s
Now, we can substitute the values into Faraday's law to calculate the mass of copper deposited:
M = (Q * Mw) / (n * F)
Substituting the known values:
M = [(2.00 A * 1200 s) * (63.55 g/mol)] / (2 * 96,485 C/mol)
Simplifying the expression:
M ≈ 0.156 g
Therefore, approximately 0.156 grams of copper is deposited on the cathode of the electrolytic cell.
To know more about electrolytic cell, visit:
https://brainly.com/question/10174059
#SPJ11
The mass of copper deposited on the cathode of the electrolytic cell is 0.787 grams. This is calculated using Faraday's law of electrolysis with the given current of 2.00 A and time of 20.0 minutes, which results in a charge of 2400 C and 0.0124 moles of copper being deposited.
To determine the mass of copper deposited on the cathode of an electrolytic cell, we need to use Faraday's law of electrolysis. According to this law, the amount of substance deposited is directly proportional to the amount of electricity passed through the cell.
First, let's convert the current to coulombs. Since the current is given as 2.00 A (amperes) and the time is 20.0 minutes, we need to convert the time to seconds. There are 60 seconds in a minute, so 20.0 minutes is equal to 20.0 × 60 = 1200 seconds.
The charge (Q) is calculated by multiplying the current (I) by the time (t): Q = I × t. Therefore, Q = 2.00 A × 1200 s = 2400 C.
Now, we need to determine the number of moles of electrons transferred. Each mole of electrons corresponds to 1 Faraday (F), which is equal to 96,485 C. Therefore, the number of moles of electrons transferred is 2400 C / 96,485 C/mol = 0.0249 mol.
Since the reaction is the reduction of Cu2+ ions to copper metal (Cu2+ + 2e- → Cu), the stoichiometry tells us that 2 moles of electrons are required to deposit 1 mole of copper.
Hence, the number of moles of copper deposited is 0.0249 mol / 2 = 0.0124 mol.
Finally, we need to calculate the mass of copper deposited. The molar mass of copper (Cu) is 63.55 g/mol. Thus, the mass (m) of copper deposited is 0.0124 mol × 63.55 g/mol = 0.787 g.
Therefore, the mass of copper deposited on the cathode is 0.787 grams.
Learn more about electrolysis
https://brainly.com/question/276473
#SPJ11
What is the pOH if the [OH-]= 0.165 M? What is the pH of this basic solution? *Please round your answer(s) to the appropriate number of significant figures. Your answer can be in standard notation or i letter "e" in place of x10.* 1 N
Answers
Answer:
78
Explanation:
Answer:
The OH would be .7825 and the basic solution is a strong base.
Explanation:
What you would do is -log(0.165) in your calculator which would give you 0.7825160065 as an answer. Im not sure what the significant figure is so I will not be rounding to that, but that is your answer for the first part.
The second part: because your pOH is a .7825, this would be consiered a strong base in the pOH because it is closer to 1 which is your base.
A reaction has an enthalpy change of − 71 kJ mol − 1 and an entropy change of − 58 J K − 1 mol − 1 . At what temperature does this exothermic reaction cease to be spontaneous?
Answers
To determine the temperature at which an exothermic reaction ceases to be spontaneous, we need to calculate the Gibbs free energy change (ΔG) and use the equation ΔG = ΔH - TΔS.
Given that ΔH = -71 kJ/mol and ΔS = -58 J/K·mol, we can calculate ΔG at different temperatures to determine the temperature at which the reaction becomes non-spontaneous.
At a temperature of 0 K, ΔG = ΔH, since TΔS = 0. Thus, ΔG = -71 kJ/mol.
As the temperature increases, TΔS becomes more negative, which means that ΔG becomes more negative, making the reaction more spontaneous.
At a certain temperature, however, ΔG will become positive, which means that the reaction is no longer spontaneous and will not proceed on its own. This temperature can be found by rearranging the equation ΔG = ΔH - TΔS to T = ΔH / ΔS, and substituting the known values for ΔH and ΔS:
T = ΔH / ΔS = -71 kJ/mol / (-58 J/K·mol) = 1230 K
So, the reaction will cease to be spontaneous at a temperature of approximately 1230 K.
Brainliest to first decent answer
What is the chemical formula for the molecule represented by the model?
CHO
C4H9O2
C4H8O
C3H8O2

Answers
The correct formula of the molecule is C4H9O2.
What is a model?The model of a compound is a representation of the molecule. It gives us an idea of what the molecule looks like as well as its molecular formula.
Looking at the structure of the compound as shown in the model in the question, the correct formula of the molecule is C4H9O2.
Learn more about molecular model:https://brainly.com/question/156574?
#SPJ1
Answer:C4H9O2.
Explanation:
1. What is the number of Neutrons in Gold (Au)?
2. What is the number of Electrons in Gold (Au)?
Answers
Answer:
Explanation:
A Gold (Au) atom has 79 protons and 79 electrons. A typical gold atom has 118 neutrons, though there are 18 other radioisotopes discovered so far.79 electrons, Gold atoms have 79 electrons and the shell structure is 2.8. 18.32. 18.1.Hope it helps:)
What is shielding and Deshielding in NMR explain with example?
Answers
"A nucleus for whom the chemical shift must have been reduced due to the addition of electron density, magnetic induction, or even other effects," according to the definition.
As electrons orbit the nucleus, they significantly change the magnetic field that even the nucleus experiences, which modifies the difference among energy levels, culminating in the spectrum. However, these adjustments are based on a specific reference as well as hence are relative.
Electron shielding is the nucleus's inhibition of valence shell electron attraction due to the existence of inner-shell electrons. Due to the spherical form of the s orbital, electrons inside an s orbital can shelter p electrons with the same energy level.
To know more about chemical shift
https://brainly.com/question/14788457
#SPJ4
If 5.17 g of an unknown substance X, which is a nonelectrolyte, lowers the freezing point of 200g of benzene ( k = 4.9 C-/m) by 0.84 C what is the molecular weight of X?
Answers
Answer:
\(M.M=150.8g/mol\)
Explanation:
Hello there!
In this case, according to the given information, it turns out firstly possible for us to set up the equation for the freezing point depression as shown below:
\(\Delta T=-i*m*Kf\)
Thus, given Kf, i (equal to 1 because it is nonelectrolyte) and the freezing point depression, we can now calculate the molality of the solution:
\(m=\frac{\Delta T}{-i*Kf}=\frac{-0.84\°C}{-1*4.9\°C/m}\\\\m=0.17mol/kg\)
Next, we calculate the kilograms of solvent by dividing the 200 g by 1000 to get 0.200 kg and thus calculate the moles of the solute X:
\(n_X=0.200kg*0.171mol/kg\\\\n_X=0.0343mol\)
Finally, the molar mass by dividing the grams by moles:
\(M.M=\frac{5.17g}{0.0343mol}\\\\M.M=150.8g/mol\)
Regards!
How many particles are in one mole?
О А.
OB. 2.066 x 1023
O C.
6.022 x 1023
O D.
6.023 × 1022
1022
3.026 x
Answers
Answer:
I think it's C. 6.022 * 1023
many hospitals use radioisotopes for diagnosis and treatment or in palliative care. three radioisotopes used in medicine are given. write the isotope symbol for each radioisotope. replace the question marks with the proper integers. replace the letter x with the proper element symbo
Answers
Radioisotopes are widely used in medicine for various purposes such as diagnosis, treatment, and palliative care. Three commonly used radioisotopes in medicine are iodine-131 (I-131), technetium-99m (Tc-99m), and cobalt-60 (Co-60).
The isotope symbol for iodine-131 is ^131I, for technetium-99m is ^99mTc, and for cobalt-60 is ^60Co. I-131 is used to diagnose and treat thyroid cancer, while Tc-99m is used in various imaging tests such as bone scans and cardiac stress tests. Co-60 is used in radiation therapy to treat cancer. The use of radioisotopes in medicine has significantly improved patient outcomes and helped in the diagnosis and treatment of various diseases.
Hospitals utilize radioisotopes for various medical purposes, such as diagnosis, treatment, and palliative care. Three commonly used radioisotopes in medicine are:
1. Technetium-99m: This radioisotope is widely used for diagnostic imaging. Its isotope symbol is 99mTc, where 99 represents the mass number (protons + neutrons) and Tc is the element symbol for Technetium.
2. Iodine-131: Iodine-131 is employed in the treatment of thyroid disorders. The isotope symbol for Iodine-131 is 131I, where 131 is the mass number and I represents the element symbol for Iodine.
3. Cobalt-60: Cobalt-60 is used in cancer therapy, specifically for external beam radiotherapy. Its isotope symbol is 60Co, where 60 denotes the mass number and Co stands for the element symbol of Cobalt.
To know about Radioisotopes :
https://brainly.com/question/28142049
#SPJ11
a student is testing a solid material to determine whether it is a pure substance or a mixture which observation would much likely indicate the material is a mixture.
Answers
Answer:
the material has two different color crystals
Explanation:
There are two types of mixture, one is homogeneous mixture and other is heterogeneous mixture. Therefore, If the material has two different color crystals, then we can see whether material is a pure substance or a mixture.
What is mixture?When two or more compounds are combined but each ingredient retains its chemical identity, the result is referred to as a mixture. In other words, there is no chemical interaction between the parts of a combination.
According to how consistent they are and how well the components' particle sizes match up, mixtures are categorized. If the material has two different color crystals, then we can see whether material is a pure substance or a mixture.
Therefore, if the material has two different color crystals, then we can see whether material is a pure substance or a mixture.
To know more about mixture, here:
https://brainly.com/question/12160179
#SPJ6
What type of energy conversion is taking place inside a flashlight when it is on?
Answers
Answer:
electrical energy
When the flashlight is turned on, the chemical energy is first transformed into electrical energy and then into light energy. CHEMICAL bonds. It is released during a chemical reaction (change). by flowing electrons (negatively charged particles).
Answer:
Electrical Energy
Explanation:
When the flashlight is turned on, the chemical energy is first transformed into electrical energy and then into light energy. CHEMICAL bonds. It is released during a chemical reaction (change). by flowing electrons (negatively charged particles).
What happens to the hydrogen and oxygen molecules when they rearrange to from water.something must be recombined.How does this happen?
Answers
When the preexisting molecular links are broken and new bonds are created between oxygen and hydrogen atoms, hydrogen molecules react strongly with oxygen molecules.
The outcome is an explosive release of energy and the formation of water since the reaction's products are at a lower energy level than its reactants.
What kind of response takes place when water is created?The most well-known instance of a synthesis reaction is the burning of hydrogen and oxygen to produce water.
What causes water to form?Most of the water that was created was in the form of vapors, which mixed with cosmic dust on the way to the earth's surface. These water vapors condensed into oceans, seas, rivers, and lakes when the world first came into existence.
To know more about hydrogen and oxygen molecules visits:-
https://brainly.com/question/12043118
#SPJ13