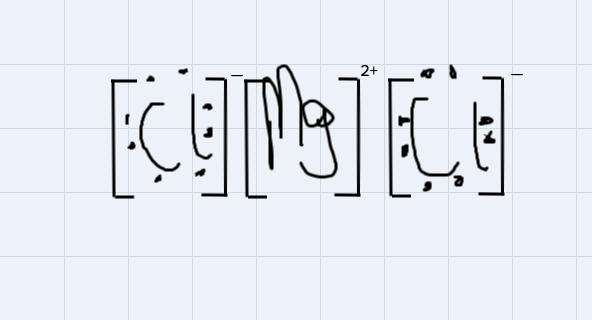
Use Lewis symbols to show how MgCl2 will be formed from Mg and Cl2.
Answers
This is a type of bonding that is formed from the from the attraction of oppositely charged ions in a compound.
For instance, MgCl2 is an ionic compound because the 2 positive ions wipossessed by the magnessium atom will attract each of the negtaive ion possessed by each of the chlorine atom to form the magnessium chloride compound
Using the Lewis symbol to demonstrate the bondng:
From the disgram, the negative ions on chlorine atoms will get attracted to the positive ions on the magnessium ion.
Related Questions
What observation about baking powder suggests that it is a mixture of substances
Answers
When water is added to both baking powder and baking soda, baking powder reacts with water to produce a gas but baking soda does not produce any gas suggesting that it is a mixture of substances.
What is baking powder?Baking powder is mixture of substances which is used in baking.
Baking powder produces a gas, carbon dioxide which is required in leavening of baking products as well as to provide smoothness and consistency in the baked products.
Baking powder is a mixture of three ingredients namely; baking soda, cream of tartar, and cornstarch.
To show that baking powder is a mixture of substances, water is added to both baking powder and baking soda. It will be observed that baking powder reacts with water to produce a gas but baking soda does not produce any gas.
In conclusion, the different observations seen when water is added to baking powder and baking soda proves that baking powder is a mixture of substances.
Learn ore about baking powder at: https://brainly.com/question/20628766
#SPJ1
Help me please and thank you

Answers
A type of alpha decay would be the one shown in the first option.
What is alpha a decay?Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle, which is a cluster of two protons and two neutrons. This results in the atomic number of the nucleus decreasing by 2, and its mass number decreasing by 4.
During alpha decay, the atomic nucleus must have sufficient energy to overcome the electrostatic repulsion between the positively charged alpha particle and the remaining nucleus. The energy released during the decay is typically very large and is carried away by the alpha particle.
Thus, an example of alpha decay is shown by option 1.
More on alpha decays can be found here: https://brainly.com/question/14081292
#SPJ1
A student states that nonmetal atoms form covalent bonds by gaining electrons until they have eight valence electrons. What is wrong with this statement?
OA. Nonmetal atoms do not usually form covalent bonds.
OB. Covalent bonds form when atoms share electrons.
O C. Nonmetal atoms form bonds until they have six valence electrons.
OD. Covalent bonds form between atoms that already have eight valence electrons.
Answers
A student states that nonmetal atoms form covalent bonds by gaining electrons until they have eight valence electrons;C Bonds are formed by nonmetal atoms up until they have six valence electrons.
What is the electrons ?
Electrons are one of the fundamental particles that make up matter. They are elementary particles of the subatomic world.The negatively charged electrons that are located outside of an atom's nucleus.. They are responsible for the electric current that flows through wires and circuits. Electrons are important components of atoms and molecules, and they are essential for forming chemical bonds. They also play an important role in the formation of magnetic fields. Electrons move around an atom's nucleus in orbitals and are able to easily jump from one atom to another, moving from one orbital to another. This makes them very important in the formation of chemical bonds between atoms.
To learn more about electrons
https://brainly.com/question/26084288
#SPJ1
How many moles of silver atoms are in 3.88x10^20 atoms of silver
Answers
Answer:
100
ddExplanation:
What is the volume of 0.58 moles
of H2 gas at STP?
Answers
Answer:
volume = 0.013 m³
Explanation:
The question asks us to calculate the volume of 0.58 moles of H₂ gas at STP.
To do this, we must use the ideal gas equation:
\(\boxed{pV = nRT}\),
where:
• p = pressure in Pa
• V = volume in m³
• n = number of moles
• R = molar gas constant (8.31 Jkg⁻¹K⁻¹)
• T = temperature in K
From the question, we know that the number of moles of H₂ is 0.58. Therefore, n = 0.58. We also know that the volume is asked for when the gas is at STP, therefore: T = 273 K, and p = 101.3 × 10³ Pa.
Using this data and the formula above, we can calculate the volume of the gas:
pV = nRT
⇒ 101.3 × 10³ × V = 0.58 × 8.31 × 273
⇒ V = \(\frac{0.58 \times 8.31 \times 273}{101.3 \times 10^3}\)
⇒ V = 0.013 m³
Therefore, the volume of the gas is 0.013 m³.
Halogens are active non metal
Answers
Answer:
True
Explanation:
They are actively non metals due to their electron configuration and number of valence electrons
8 vocabulary words related to energy sources
Answers
Answer:
Solar energy. Biofuel, Nuclear power, Hydro power, Geothermal energy, Fossil, Gasoline, sun
Mechanical waves need to travel through a medium like solid, liquid, and gas.
TRUE or False
Answers
Answer:
Mechanical waves are waves that must go through a medium in order to propagate. Mechanical waves can also be transmitted through solids, so the medium doesn't have to be a gas or a liquid.
Explanation:
Hope this helps! Brainliest would be appreciated. :)
In step 3, which of the water drops was the flattest and widest? what does this mean about the attraction of the molecule to the surface?
Answers
The flattest and widest water drop was the one with the highest surface tension, indicating the highest attraction of the molecule to the surface.
This is because the molecules have a stronger cohesive force, which is the force of attraction between the molecules of the same substance, and a higher surface tension, which is the force of attraction between the molecule and the surface. The higher the cohesive force, the more the molecules will be attracted to each other and the higher the surface tension, the more the molecules will be attracted to the surface. Therefore, the flattest and widest water drop indicates the strongest attraction of the molecule to the surface.
learn more about cohesive force Refer:brainly.com/question/24666570
#SPJ4
Copper-64 is used in the form of copper acetate to study brain tumors. it has a half-life of 12.8 h. if you begin with 15.0 mg of 64 cu-labeled copper acetate, what mass remains after 64 hour?
Answers
After 64 hours, approximately 0.47 mg of Copper-64 labeled copper acetate remains.
To solve this problem, we need to use the concept of half-life, which is the amount of time it takes for half of the radioactive substance to decay.
First, we need to determine how many half-lives have passed in 64 hours. Since the half-life of Copper-64 is 12.8 hours, we can divide 64 by 12.8 to get 5.
This means that after 64 hours, Copper-64 has undergone 5 half-lives.
To determine the amount of Copper-64 that remains, we can use the following equation:
Final mass = initial mass x (1/2)^(number of half-lives)
Plugging in the given values, we get:
Final mass = 15.0 mg x (1/2)^5
Final mass = 15.0 mg x 0.03125
Final mass = 0.46875 mg = 0.47 mg
Therefore, after 64 hours, only 0.47 mg of Copper-64 labeled copper acetate remains.
Learn more about half-life : https://brainly.com/question/1160651
#SPJ11
determine the oxidation state of the metal species in the complex ion. [fe(cn)(co)5]2
Answers
The oxidation state of the metal species, Fe, in the complex ion [Fe(CN)(CO)5]2- is -1.
In order to determine the oxidation state of the metal species (Fe) in the complex ion [Fe(CN)(CO)5]2-, we first need to examine the individual ligands and their respective charges.
The ligands in the complex ion are cyanide (CN-) and carbonyl (CO). Cyanide is a monodentate ligand with a charge of -1, while carbonyl is a neutral ligand with a charge of 0. Since there is only one cyanide ligand, the total charge from cyanide is -1. There are five carbonyl ligands, but as they are neutral, they do not contribute any charge to the complex.
Now, we know the overall charge of the complex ion is -2, as indicated by the 2- in the formula. Considering the charges from the ligands, we can set up an equation to find the oxidation state of the metal species, Fe:
Fe + (-1) + 5(0) = -2
Simplifying the equation, we get:
Fe - 1 = -2
Adding 1 to both sides of the equation, we arrive at:
Fe = -1
Learn more about oxidation state here :-
https://brainly.com/question/31688257
#SPJ11
Explain how the experimental molecular mass would have been affected (too high, too low or unchanged) if the mass of the flask had been measured with condensed vapor and residual water on the outside. Please support your answer.
Answers
If the flask already has water, your measure is going to be higher than expected and MW is going to be higher too.
At first sight, we need to assume 1 atm as atmospheric pressure, probably forgot to write down this value in the Lab. By doing this, we got all values to calculate the MW.
So, for trial 1:
MW = [0.36 g x (0.082 atm x L / K x mol) x 301 K] / (1 atm x 0.125 L) = 71.08 g/mol
Trial 2:
MW = [0.38 g x (0.082 atm x L / K x mol) x 301 K] / (1 atm x 0.125 L) = 75.53 g/mol
Average MW = 73.31 g/mol
Learn more about atmospheric pressure here: https://brainly.com/question/87231
#SPJ4
Calculate the pOH and the pH of a 5.0 x 10-2 M solution of NaOH.
Answers
Answer:
pOH = 1.3, pH = 12.7
Explanation:
Since NaOH is a strong base, it will completely ionize; further, since it completely ionizes, our hydroxide concentration (a product of the ionization) will be the same as the given concentration of NaOH.
NaOH -> Na⁺ + OH⁻, [OH⁻] = 5.0 x 10^-2 M
pOH is the negative log of the hydroxide concentration, so plug our hydroxide concentration in:
pOH = -log[OH⁻] = -log[5.0 x 10^-2 M] = 1.3
Since pH + pOH = 14, we can plug in pOH and solve for pH:
pH + 1.3 = 14
pH = 14 - 1.3 = 12.7
Thus, our pOH = 1.3 and pH = 12.7.
electrons coming off the cathode in an x-ray machine are focused to hit a small area of the anode called the: a. tube. b. target. c. transformer. d. focusing plate.
Answers
Electrons coming off the cathode in an X-ray machine are focused to hit a small area of the anode called the target.
In an X-ray machine, a cathode emits a stream of electrons, which are accelerated toward the anode. The anode consists of a small area known as the target, which is specifically designed to interact with the high-speed electrons. When the electrons strike the target, they undergo a rapid deceleration, resulting in the production of X-rays.
The target area on the anode is typically made of a high atomic number material, such as tungsten or molybdenum, that is capable of withstanding the high heat generated during the X-ray production process. The small focal spot on the target helps to ensure that the X-rays are emitted from a specific location, allowing for better control and precision in directing the X-ray beam.
In summary, the electrons from the cathode are focused to hit a small area of the anode known as the target, where X-rays are generated through the interaction of the electrons with the target material.
To learn more about x-ray machine, here
https://brainly.com/question/31288702
#SPJ4
In which of the following states of matter are the particles very far apart and
move freely?
O solids only
O solids and liquids only
O solids, liquids, and gases
O gases only
Answers
Answer:
Gas
Explanation:
In a gas, particles are in consistent straight-line movement. The motor energy of the atom is more prominent than the alluring power between them, hence they are a lot farther separated and move unreservedly of one another.
Answer: Gases only
Explanation: because there are gas all around us. You can walk though the air with slamming into it like its a brick wall. that's because the particles are far apart allowing it to move freely.
Hopefully this helps.
How many moles of Ba(OH)2 are present in 205 mL of 0.600 M Ba(OH)2?
Answers
Answer:
Explanation:
0.180 moles of Ba(OH)2 are present.
Answer:
\(\boxed {\boxed {\sf 0.123 \ mol \ Ba(OH)_2}}\)
Explanation:
Molarity is a measure of concentration in moles per liter. It is calculated using the following formula:
\(molarity= \frac{moles \ of \ solute}{liters \ of \ solution}\)
The molarity of the solution is 0.600 M Ba(OH)₂. 1 molar (M) is also equal to 1 mole per liter, so the molarity is 0.600 moles of Ba(OH)₂ per liter.
The volume of the solution is 205 milliliters, however, we need to convert the volume to liters. Remember that 1 liter contains 1000 milliliters.
\(\frac { 1 \ L}{1000 \ mL}\)\(205 \ mL * \frac { 1 \ L}{1000 \ mL} = \frac {205}{1000} \ L = 0.205 \ L\)Now we know the molarity and volume, but the moles are still unknown.
molarity = 0.600 mol Ba(OH)₂/ L moles of solute = x liters of solution = 0.205 LSubstitute these values into the formula.
\(0.600 \ mol \ Ba(OH)_2 /L = \frac{x}{0.205 \ L}\)
We are solving for x or the moles of solute, so we must isolate the variable. It is being divided by 0.205 liters. The inverse of division is multiplication. Multiply both sides by 0.205 L.
\(0.205 \ L *0.600 \ mol \ Ba(OH)_2 /L = \frac{x}{0.205 \ L} * 0.205 \ L\)
\(0.205 \ L *0.600 \ mol \ Ba(OH)_2 /L =x\)
The units of liters cancel.
\(0.205 *0.600 \ mol \ Ba(OH)_2 =x\)
\(0.123 \ mol \ Ba (OH)_2 = x\)
The original measurements had at least 3 significant figures. Our answer currently has 3 sig figs, so we don't need to round.
There are 0.123 moles of barium hydroxide.
What would be the most effective way for a scientist to get an idea of the actual age of a rock?
Answers
Answer:
Radiometric dating
Explanation:
Radiometric dating would be the most effective way since it is a technique that can establish the age of objects older than a few thousand years.
If 50 mL of a 1:20 w/v solution is diluted to 1000 mL, what is the ratio strength (w/v)?
Answers
The ratio strength (w/v) of the diluted solution is 1:1000 or 0.1% w/v.
The original solution is a 1:20 w/v solution, which means that for every 1 gram of solute, there is 20 mL of solution. Using this information, we can calculate the amount of solute in the original 50 mL of solution:
1 gram / 20 mL = x grams / 50 mL
x = 2.5 grams of solute
When this 50 mL of solution is diluted to 1000 mL, the amount of solute remains the same, but the volume of the solution increases. The new ratio can be calculated by dividing the weight of the solute by the volume of the solution:
2.5 grams / 1000 mL = 0.0025 grams/mL
Converting this to a percentage w/v:
0.0025 grams/mL x 100 = 0.25% w/v
Therefore, the ratio strength is 1:1000 or 0.1% w/v.
To know more about diluted solution, refer here:
https://brainly.com/question/1416865#
#SPJ11
What is the relationship between mole, Avogadro number and mass?
a.1mole=6.022 × 1023 atoms and molecules= g atomic/molecular mass
b. 1mole =6.022 × 1023 atoms and molecules /1 g atomic/molecular mass
c. 2mole=6.022 × 1023 atoms and molecules=1 g atomic/molecular mass
d. 1mole < 6.022 × 1023 atoms and molecules=1 g atomic/molecular mass
Answers
Answer:
The mass of one mole of a substance is equal to that substance's molecular weight. ... water is 18.015 atomic mass units (amu), so one mole of water weight 18.015 grams. ... Avogadro's number is a proportion that relates molar mass on an atomic ... one molecule of water (H2O), one mole of oxygen (6.022×1023 of O atoms)
. Now Sara comes along, and she is the exact same size as you. However, she is even stronger than you are! When she pulls you in the wagon, she pulls with a greater force than when you pull her. Now who is in the wagon when it has the greatest acceleration? Explain, using Newton's second law. Sara would win because she is stronger but we have the same force.
Answers
Answer:
See explanation
Explanation:
According to Newton's second law, the rate of change of momentum is proportional to the impressed force.
Mathematically;
Ft = mv - mu
Where;
F = Force
t = time
m= mass
v = final velocity
u = initial velocity
Since the mass is the same, the equation reduces to;
Ft = m(v-u)
So
F= m(v-u)/t
but
(v-u)/t = acceleration (a)
F = ma
a = F/m
Since the mass is the same because Sarah is the same size as i am but she pulls with a greater force, it follows that i will be the one in the wagon when the wagon has the greatest acceleration because the force that Sarah applies when pulling me is greater than the force i will apply when pulling her.
A farmer wants to build a pond for her cows. What step must she take in order to build the pond?
A. Dig into the zone of aeration.
B. Place a permeable material on the ground.
C. Keep the soil moist.
D. Place an impermeable material on the ground.
Answers
Answer:
Option A:
Dig into the zone of aeration
Explanation:
Within the lithosphere of the earth's surface, the zone of aeration is the zone directly above the water table, with a lot of pore spaces within the rocks. These pore spaces contain air and water, which can be used to water the cows.
Once the zone of aeration has been dug into, the farmer can tap into the underground reserve of water, which is stored within the pores of rocks. This water can now sip into the hole that was dug up by the farmer, forming a pond for the cows.
The isomerization of cyclopropane to propylene is a first-order process with a half-life of 19 minutes at 500oc. The time it takes for the partial pressure of cyclopropane to decrease from 1. 0 atmosphere to 0. 125 atmosphere at 500oc is closest to.
Answers
The isomerization of cyclopropane to propylene is a first-order process with a half-life of 19 minutes at 500oc. The time it takes for the partial pressure of cyclopropane to decrease from 1. 0 atmosphere to 0. 125 atmosphere at 500oc is closest to 57 minutes.
First, we must get the rate constant.
k= 0.693/ 19 = 0.0365 min⁻¹
Now we need to figure out how long decay takes.
First order kinetics' expression for the rate law is provided by:
t= (2.303/k) log a/(a-x)
where,
Rate constant = k
t = time it took for decay to occur.
Initial reactant pressure equals 1.0 atm, or a.
After the process of decay, a - x equals 0.125 atm of pressure.
After entering all the values into the equation above, we get
t= (2.303/0.0365) log 1/(1-0.125)
t = 57 min
Learn more about first order reaction at https://brainly.com/question/29788906
#SPJ4
what is a good definition of the atomic radius? Note: you cannot use 'radius' in your definition
Answers
Answer:
the distance between the electron and nucleus
* GIVING BRAINLIEST*
3Ca+2 FeCl3 -> 3CaCl2 + 2Fe
Calcium metal + Iron Chloride -> Calcium Chloride + Iron metal
Identify the reason that atoms react with each other.
Answers
i think its double replacement if i'm not mistaken
what is the compound name for the ION SbBO3. Also can someone please tell me what the difference is between just a regular molecule and a ion. And how it changes formulas? Thank you so much!
Answers
Answer:
Antimony Borate
Explanation:
The compound name for the ion SbBO3
That would be Antimony borate
Initially we have the combination of antimony and the borate ion
Antimony has the ion Sb3+ while the borate have the ion BO3(3-)
According to IUPAC naming rules, the 3 on both ions will cancel out during combination to form a single molecule.
The difference between just a regular molecule and an ion is that while a molecule does not carry charge, an ion is charged. The positive or negative charge on an ion is what makes it different from a molecule which is not charged. Also, there must be a combination of a positive and a negative ion before we can have the formation of a molecule
Now, how it changes formula?
Although there are extensive rules but basically, a molecule is formed if there is a combination of ions.
Now, how an ion changes form in a molecule depends on the charge on the other ion
A positive ion most times come in contact with a negative ion to form a molecule.
Now, if we have the same magnitude of charge in both positive and negative sides, the charges will cancel out.
This is in the case of Antimony Borate where we had a magnitude of 3 on the positive and negative sides.
So they cancel out
In a case where this is not the case such as in the case of calcium and borate ion,
We have Calcium as Ca2+ and the borate ion as BO3(3-)
What happens here is that they switch ions and we have;
Ca3(BO3)2
Hope these helps!
Thank you
4. For a typical vertebrate cell with a membrane potential of 0.050 V (inside negative), what is the free-energy change for transporting 1 mol of Ca+2 from the cell into the blood at 37 °C? Assume the concentration of Ca+2 inside the cell is 145 mM and in blood plasma it is 25 mM. Does this transport take place spontaneously or not? (R= 8.315 J/mol.K)
Answers
Free energy change for transporting Ca2+ ions is calculated as follows:∆G = RT ln ([Ca2+]outside/[Ca2+]inside)∆G = 8.315 J/mol.K x 310 K x ln (25 mM/145 mM) = -15,400 J/mol.
Here, ∆G is negative, which implies that Ca2+ ions transport spontaneously from the cell to blood. This is because the free energy of the system decreases when Ca2+ ions move from high concentration to low concentration. Therefore, transporting Ca2+ ions is energetically favorable.
To know more about calculated visit:
https://brainly.com/question/30781060
#SPJ11
Which of the following is NOT an example of matter?
O All of these are examples of matter
O air
O gold
O apple juice
O a rose bush
Answers
Answer: All of these are examples of matter
Explanation:
Matter is any substance that has mass and takes up space by having volume. All examples have mass (including air) and take up space, so the answer is, "All of these are examples of matter."
Hope this helps.
Help me with this pls

Answers
In 1 angle AOD is an obtuse angle and is 110° and angle GOD is an acute angle and it is 70°.
What is an acute angle and an obtuse angle?90 degrees is the standard for a right angle. Any angle that is less than 90 degrees is called acute, and any angle that is larger than the 90 degrees is called obtuse.
How to use a Protractor for acute angle?Follow the instructions below to use a protractor to measure an angle. Align the angle's vertex with the protractor's dot in the center. Set the protractor such that one angle's side is parallel to 0 degrees. Find the point where the angle's opposite side crosses the number scale by reading the protractor.
To know more about angles visit
https://brainly.com/question/28451077
#SPJ1
how many moles of manganese are in 1 mole of manganese(iv) permanganate
Answers
Answer:
4 moles...................
how is water different from hydrogen peroxide even though both compounds are composed of only hydrogen and oxygen?
Answers
While both hydrogen and oxygen are present in water (\(H_2O\)) and hydrogen peroxide (\(H_2O_2\)), their structural differences give rise to differences in their chemical and physical characteristics.
The amount of oxygen atoms in each molecule of water and hydrogen peroxide is the key distinction between the two substances. Hydrogen peroxide has two oxygen atoms, compared to one in water. Hydrogen peroxide differs from water in both chemical and physical characteristics due to its distinct molecular structure.
A very reactive substance, hydrogen peroxide easily conducts chemical processes, including breakdown or reduction, to produce other molecules. The additional oxygen atom in hydrogen peroxide, which results in an unstable structure and a great propensity to react with other molecules, is the cause of this reactivity.
Learn more about water and hydrogen peroxide at
https://brainly.com/question/14294502
#SPJ4