The structure of matter refers to its _____.
Select one:
a.
composition
b.
measurements
c.
behavior
d.
reactions
Answers
The structure of matter refers to its composition.
a. compositionRelated Questions
In the formation of SO2 and SO3 the ratio of the weight of oxygen which combines with 10kg of sulphur is ?
Answers
Explanation:
So,0.3125 * 10 ^3 moles of Sulphur combines with 0.3125 * 10^3 moles of Oxygen to from SO2. Therfore mass ratio of SO2 : SO3 = 10 : 15 = 2:3.
If an atmosphere consisting of an ideal gas were indeed homogeneous (constant density) all the way to the top at z = H, find the temperature at that top. Could such an atmosphere actually exist, even in principle? Why or why not?
Answers
The temperature at the top of an atmosphere consisting of an ideal gas with constant density can be found using the ideal gas law, which states that PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature.
Assuming the density of the gas is constant throughout the atmosphere, we can express the pressure as P = ρgh, where ρ is the density, g is the acceleration due to gravity, and h is the height.
Since the atmosphere is homogeneous, the pressure at the top (P_top) is the same as the pressure at the bottom (P_bottom), so we have P_top = P_bottom = ρgh.
Using the ideal gas law, we can write ρghV = nRT, where V is the volume of the gas.
Assuming the volume of the gas is constant, we can cancel out V, and rearranging the equation, we get T_top = (nRT_bottom)/(nR) = T_bottom.
Therefore, the temperature at the top of the atmosphere would be the same as the temperature at the bottom.
In practice, however, it is not possible for an atmosphere to have constant density all the way to the top. As we go higher in the atmosphere, the density decreases due to decreasing pressure and temperature. This is because gravity becomes weaker at higher altitudes. Therefore, in reality, the temperature would decrease as we move higher in the atmosphere.
So, while a homogeneous atmosphere with constant density all the way to the top cannot exist in practice, it can be considered as an idealized scenario for understanding certain aspects of atmospheric behavior.
Learn more about ideal gas law, here :-
https://brainly.com/question/30458409
#SPJ11
What two gases are cycled through ecosystems?
Answers
\(\huge\tt \blue{❥Answer}\)
Photosynthesis converts carbon dioxide gas to organic carbon, while respiration cycles the organic carbon back into carbon dioxide gas.
______________________________
~ ʜᴀᴘᴘʏ ʟᴇᴀʀɴɪɴɢ ~
Sodium cyanide reacts with 2‑bromobutane in dimethylsulfoxide to form a substitution product. Draw the substitution product formed when this enantiomer reacts with nacn in dmso. Clearly indicate stereochemistry by drawing a wedged bond, a dashed bond, and two in‑plane bonds per each chiral carbon. Then identify the configuration of the starting material and the product.
Answers
Reactant has S -configuration while Product has R configuration at chiral carbon of DMSO.
The enantiomers of a chiral substance are referred to by the terms "right hand" and "left hand" in naming. The stereo-centers have a R or S designation.
The stereocenter arrangement is referred to as S ("Sinister" Latin Meaning "left") if the arrow points counterclockwise (left after leaving the 12 o'clock position). The stereocenter is designated R ("Rectus" Latin for "right") if the arrow, on the other hand, points in a clockwise direction (right upon leaving the 12 o'clock position). The name of the target enantiomer is then preceded by the letters R or S in parentheses.
To know more about R and S configuration visit :https://brainly.com/question/13664402
#SPJ1
a polystyrene molecule has a molar mass of 18,000 g/mol. calculate the number of monomer units (the degree of polymerization) for this molecule.
Answers
This polystyrene molecule is roughly 173 percent polymerized.
What is the polymerization formula?Multiply the molecular weight of the monomer by the polymer's molecular mass. Tetrafluoroethylene, for instance, has a molecular mass of 1,20,000; its degree of polymerization is computed as 1,20,000 / 100 = 1,200. Hence, 1,200 is the degree of polymerization.
A polymer called polystyrene is created by repeating units of styrene monomers. By dividing the molar mass of the styrene monomer by the quantity of monomer units (degree of polymerization) present in the polymer, one may get the molar mass of polystyrene, which is 18,000 g/mol.
Let's call the styrene monomer's molar mass M. Then, we may create the following equation:
18,000 g/mol = M × n
where n is the degree of polymerization (the number of monomer units).
Rearranging the equation, we can solve for n:
n = 18,000 g/mol ÷ M
The periodic chart shows the molar mass of styrene as the total of the atomic masses of its component elements as follows:
M(styrene) = 104.15 g/mol (28.05 g/mol for carbon x 8 + 1.01 g/mol for hydrogen x 8)
When we enter this number into the equation, we obtain:
n = 18,000 g/mol ÷ 104.15 g/mol ≈ 173
To know more about polystyrene visit:-
https://brainly.com/question/15326238
#SPJ1
In the first reaction of the citric acid cycle, oxaloacetate, which contains ______ carbons, is combined with acetyl-CoA, which contains ______ carbons in its acetyl group, to produce citric acid, which contains ______ carbons.
Answers
Oxaloacetate contains 4 carbons. Acetyl-CoA contains 2 carbons in its acetyl group. Citric acid contains 6 carbons. In the first reaction of the citric acid cycle, acetyl-CoA combines with oxaloacetate to form citrate.
The acetyl group of acetyl-CoA is transferred to oxaloacetate, and a molecule of CoA is released. The resulting citrate molecule has 6 carbons. The citric acid cycle is a series of chemical reactions that occur in the mitochondria of cells. The cycle is responsible for the oxidation of acetyl-CoA, which produces energy in the form of ATP. The cycle also produces NADH and FADH2, which are used in the electron transport chain to produce more ATP. The citric acid cycle is a critical part of cellular respiration, and it is essential for the production of energy.
To know more about citric acid cycle, click here:-
https://brainly.com/question/11238674
#SPJ11
What would be the molar ratio in the production of hydrogen iodide gas fromhydrogen and iodide gases, as shown in the following chemical reaction: *H2 (g) + 12 (9)2HI (g)1:1:12:1:21:1:22:1:1
Answers
The chemical reaction of the formation of hydrogen iodide is given:
H2(g) + I2(g) = 2HI (g)
As you can see from the equation, only 1 mole of hydrogen and 1 mole of iodine react to form 2 moles of hydrogen iodide
Three elements in the same period are listed in order of decreasing atomic radius. Which of the following is an appropriate explanation for the non-metal in the list having the smallest atomic radius
Answers
The appropriate explanation for the non-metal is that the higher effective nuclear charge less will be the atomic radius.
Atomic radius typically decreases during a period from left to right. There are a few little outliers, such how the oxygen radius is a tiny bit bigger than the nitrogen radius. Protons are gradually added to the nucleus at the same time that electrons are gradually added to the main energy level. The enhanced positive charge of the nucleus gradually attracts these electrons closer to it. The size of the atoms shrinks as the strength of attraction between nuclei and electrons grows. Due to electron-electron repulsions that would otherwise result in the atom's size expanding, the effect becomes less pronounced as one proceeds further to the right in a period.
Learn more about nuclear charge
brainly.com/question/13664060
#SPJ4
Given the following reaction in acidic solution: Fe2+ (aq) + Cr2O72- (aq) ? Fe3+ (aq) + Cr3+ (aq). What is the coefficient of water in the balanced reaction?
Answers
In the reaction: Fe²⁺(aq) + Cr₂O₇²⁻(aq) → Fe³⁺(aq) + Cr³⁺(aq), the coefficient of water in the balanced reaction is 7.
To find the coefficient of water in the balanced reaction Fe²⁺(aq) + Cr₂O₇²⁻(aq) → Fe³⁺(aq) + Cr³⁺(aq) in an acidic solution, follow these steps:
1. Determine the oxidation states of each species:
Fe²⁺ has an oxidation state of +2
Cr₂O₇²⁻: Cr has an oxidation state of +6, and O has an oxidation state of -2
Fe³⁺ has an oxidation state of +3
Cr³⁺ has an oxidation state of +3
2. Identify the species being oxidized and reduced:
Fe²⁺ is being oxidized to Fe³⁺ (oxidation state increases from +2 to +3)
Cr₂O₇²⁻ is being reduced to Cr³⁺ (oxidation state decreases from +6 to +3)
3. Balance the atoms and charges through half-reactions:
Oxidation half-reaction: Fe²⁺ → Fe³⁺ + e⁻
Reduction half-reaction: Cr₂O₇²⁻ + 14H⁺ + 6e⁻ → 2Cr³⁺ + 7H₂O
4. Balance the number of electrons transferred in both half-reactions:
Oxidation: 6Fe²⁺ → 6Fe³⁺ + 6e⁻
Reduction: Cr₂O₇²⁻ + 14H⁺ + 6e⁻ → 2Cr³⁺ + 7H₂O
5. Add the balanced half-reactions to obtain the balanced overall reaction:
6Fe²⁺ + Cr₂O₇²⁻ + 14H⁺ → 6Fe³⁺ + 2Cr³⁺ + 7H₂O
Therefore, the coefficient of water in the balanced reaction is 7.
To know more about the coefficient of water click here:
https://brainly.com/question/28218215
#SPJ11
What were the first 5 planets that were observed? Why were those the only ones? Help pls!
Answers
the half life of a given radioactive isotope is 5 minutes how many grams of 48 gram sample would remain after 20 minutes have elapsed?
Answers
Answer:
3
Explanation:
the half life of a radioactive isotope is the amount of time one half of the isotope takes to decay
after 5 minutes the mass will be 24
after 10 minutes the mass will be 12
after 15 minutes the mass will be 6
after 20 minutes the mass will be 3
true or false? pth and 1,25(oh)2d (vitamin d) are the principal hormones involved in the normal physiologic regulation of calcium homeostasis.
Answers
PTH and 1,25(OH)2D are the primary hormones responsible for maintaining calcium balance in the body, playing crucial roles in regulating calcium levels.
PTH and 1,25(OH)2D work in tandem to regulate calcium levels in the body. PTH increases calcium release from bones and enhances calcium reabsorption by the kidneys, while active vitamin D promotes calcium absorption from the intestines. This coordinated action of PTH and 1,25(OH)2D helps maintain the normal physiologic regulation of calcium homeostasis.
Parathyroid hormone (PTH) is released by the parathyroid glands in response to low blood calcium levels. PTH acts on the bones, kidneys, and intestines to increase calcium levels. It stimulates the release of calcium from the bones, enhances the reabsorption of calcium by the kidneys, and promotes the production of active vitamin D.
For more information on calcium metabolism visit: brainly.com/question/15269453
#SPJ11
SURELY SOMEONE HELP it’s urgent plllss I’ll brainlist u/5 star!!! answer the ones u know. :)
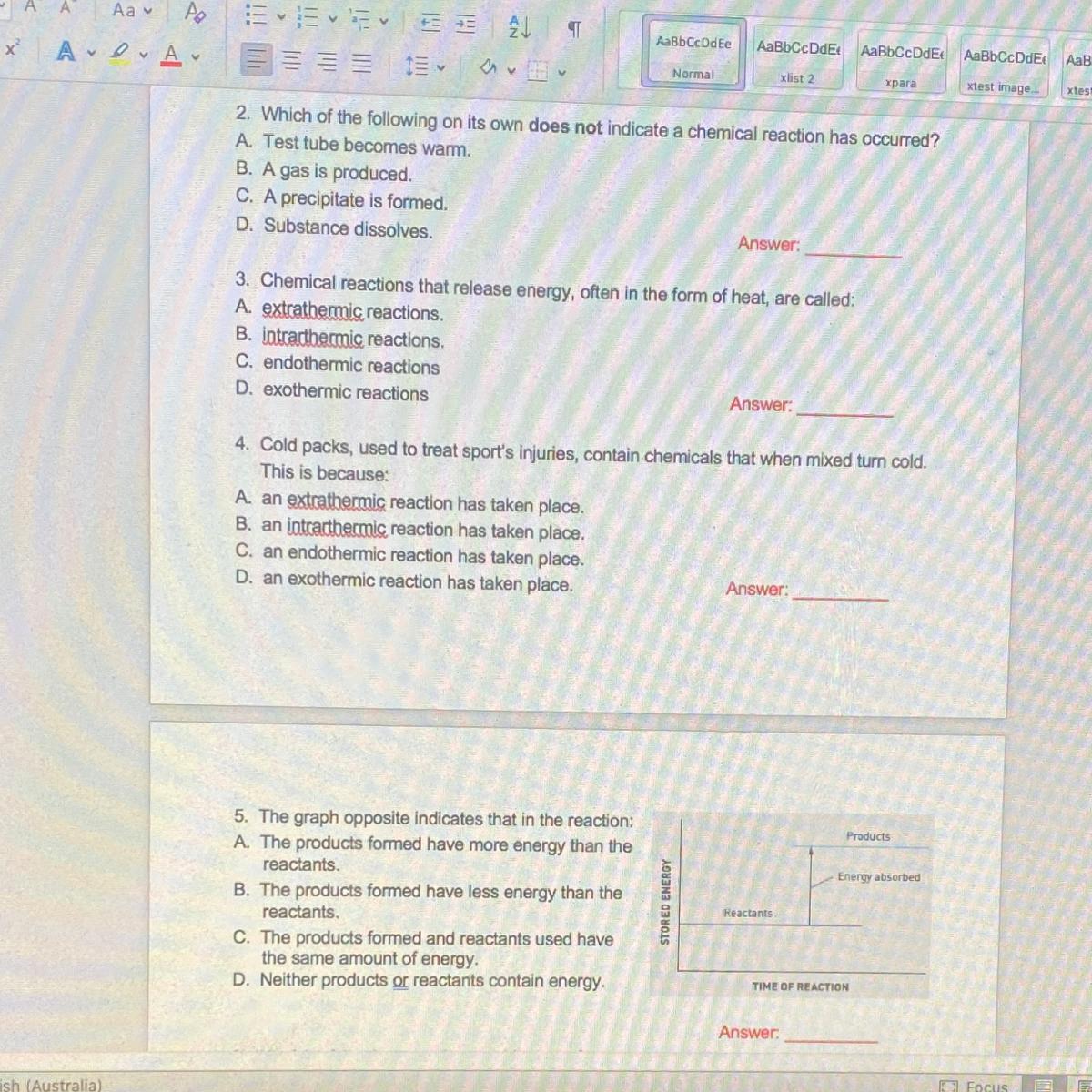
Answers
Answer:
3 exothermic reaction. only that much
Hi,
These are the answers.
• Question 2. C , Substance dissolves
• Question 3. D , Exothermic reaction
• Question 4. C , Endothermic reaction takes place
• Question 5. A , the products formed has more energy than reactants
Hope it helps you... pls mark brainliest if it helped you
Assume that a scientist is measuring mass in an experiment. the balance she is using is not set a zero so it always measures mass a little too high what type of error does this cause in her data
Answers
In the given query, if balance is not set on a zero, the error caused by the balance being off-zero is termed as a systematic error.
Error is any preventable event that is caused by a person due to lack of knowledge, lack of skills etc.
Systematic errors are usually caused by faulty equipment or incorrect calibration of the instruments used in the experiment. They can be minimized by calibrating the instruments regularly and ensuring that they are functioning correctly before the experiment begins.
This type of error affects the accuracy of the measurements, as it causes all measurements to be shifted in the same direction.
In this case, the balance measures mass a little too high, which means that all measurements will be overestimated by the same amount.
Therefore, the systematic error caused by the balance being off-zero can have a significant impact on the accuracy of the measurements.
Learn more about error here:
https://brainly.com/question/31777604
#SPJ12
What are the half-reactions for a galvanic cell with Zn and Mg electrodes?
Answers
the half-reactions
cathode : Zn²⁺ (aq) + 2e⁻ ---> Zn (s)
anode : Mg (s) → Mg²⁺ (aq) + 2e−
a balanced cell reaction
Zn²⁺(aq) + Mg(s)→ Zn(s) + Mg²⁺ (aq)
Further explanationGiven
Zn and Mg electrodes
Required
The half-reactions for a galvanic cell
Solution
To determine the reaction of a voltaic cell, we must determine the metal that serves as the anode and the metal that serves as the cathode.
To determine this, we can either know from the standard potential value of the cell or use the voltaic series
1. voltaic series
Li-K-Ba-Ca-Na-Mg-Al-Mn- (H2O) -Zn-Cr-Fe-Cd-Co-Ni-Sn-Pb- (H) -Cu-Hg-Ag-Pt-Au
The more to the left, the metal is more reactive (easily release electrons) and the stronger reducing agent
So the metal on the left will easily undergo oxidation and function as anode
Since Mg is located to the left of Zn, then Mg functions as anode and Zn as a cathode
2. Standard potentials cell of Mg and Zn metals :
Mg2+ + 2e– → Mg E° = -2,35 V
Zn2+ + 2e– → Zn E° = -0,78 V
The anode has a smaller E°, then Mg is the anode and Zn is the cathode.
Answer:
Explanation:help
Calculate the number of moles of Na+ ions in 25g of sodium carbonate?
Answers
Explanation:
First, we need to calculate the number of moles of sodium carbonate we have in a 25 g sample. To calculate this, we will
find the molar mass of sodium carbonate (Na2CO3):
⇒ 2 × Molar mass of sodium + Molar mass of carbon + 3×molar mass of oxygen
⇒ 2 × 23 + 12 + 3 × 16
⇒ 46 + 12 + 48
⇒ 106g/mol
Thus, the molar mass of Na2CO3 is 106g/mol.
Therefore, number of moles = 25 ÷ 106
=> 0.2358 mol
Now, we know that every mole of Na2CO3 have 0.2358 moles of Na+ ions. Hence, total moles of Na2CO3 is 0.4716 moles
Number of ions present = 6.022 × 1023 × 0.4716 mol = 2.84 × 1023ions
The molar mass of sodium carbonate is 106 g/mol. One mole of sodium carbonate gives 2 Na+ ions. Thus number of moles in 25 g of sodium carbonate is 0.47 moles.
What is sodium ions?Sodium ion Na+ is formed by the lose of one electron from the sodium metal. Sodium has one valence electron and it will reacts with other atoms by donating this electron and gaining a positive charge.
The molecular formula of sodium carbonate is Na₂CO₃. Therefore, one mole of sodium carbonate will give two moles of Na+ ions. The molar mass of sodium carbonate is 106 g/mol.
The number of moles of 25 g of sodium carbonate is calculated as follows:
Number of moles = 25 /106
= 0.235 moles.
It is clear that one mole of Na₂CO₃ gives two moles of sodium ions.Then, number of moles of sodium ions given by 0.235 moles of sodium carbonate is calculated as follows:
number of moles of Na+ = 0.235 × 2
= 0.47 moles
Hence, the number of moles of Na+ ions produced by 25g of sodium carbonate is 0.47 moles.
To find more about sodium carbonate, refer the link below:
https://brainly.com/question/28901831
#SPJ2
how many moles of cubr are contained in 244 ml of 0.135 m cubr solution? the density of the solution is 1.22 g/ml. reddit
Answers
Answer:
idc
Explanation:
idc looooooooooool
How many grams of CO2 form when 7.50 g of C2H5OH are produced?
Answers
To determine the grams of CO2 formed when 7.50 g of C2H5OH (ethanol) is produced, we need to consider the balanced chemical equation for the combustion of ethanol:
C2H5OH + 3O2 -> 2CO2 + 3H2O
From the balanced equation, we can see that for every 1 mole of C2H5OH, 2 moles of CO2 are produced. We can use the molar mass of ethanol and the molar mass of CO2 to calculate the grams of CO2 produced.
The molar mass of C2H5OH is calculated as follows:
(2 x molar mass of C) + (6 x molar mass of H) + molar mass of O
(2 x 12.01 g/mol) + (6 x 1.008 g/mol) + 16.00 g/mol = 46.07 g/mol
Now we can set up a proportion to calculate the grams of CO2:
(7.50 g C2H5OH) / (46.07 g/mol C2H5OH) = (x g CO2) / (44.01 g/mol CO2)
Cross-multiplying the proportion:
7.50 g C2H5OH * (44.01 g/mol CO2) = 46.07 g/mol C2H5OH * x g CO2
Simplifying the expression:
x = (7.50 g C2H5OH * 44.01 g/mol CO2) / 46.07 g/mol C2H5OH
Calculating the result:
x ≈ 7.17 g CO2
Therefore, approximately 7.17 grams of CO2 are formed when 7.50 grams of C2H5OH are produced.
Balance the following chemical equations.
a) Ba Cl2 + H2SO4 BaSO4 + HCl.
b) Calcium hydroxide + Carbon dioxide Calcium carbonate + Water.
c) Aluminum + Copper chloride Copper + Aluminum chloride
d) Sulphur dioxide + Oxygen Sulphur trioxide
e) NH3+ CuO Cu + N2 + H2O
Answers
Answer:
See the explanation
Explanation:
In this question we have to take into account that we have to get the same amount of atoms on both sides, so:
Reaction 1
\(BaCl_2~+~H_2SO_4~=>~BaSO_4~+~2HCl\)
We have 1 Ba, 2 Cl, 2 H and 4 O on both sides
Reaction 2
\(Ca(OH)_2~+~CO_2~=>~CaCO_3~+~H_2O\)
We have 1 Ca, 2O, 2 H and 1 C on both sides
Reaction 3
\(2Al~+~3CuCl_2~=>~2 AlCl_3~+~3Cu\)
We have 2 Al, 3 Cu, and 3 Cl on both sides
Reaction 4
\(2SO_2~+~O_2~=>~2SO_3\)
We have 2 A and 6O on both sides
Reaction 5
\(2NH_3~+~3CuO~=>~3Cu~+~N_2~+~3H_2O\)
We have 2 N, 3 H, 3 Cu and 3O on both sides.
I hope it helps
what is the solute when stirring salt in water until the salt disappears?
Answers
Answer:
The solute is the substance being dissolved.
The solvent is the substance dissolving the solute.
Therefore, the salt is the solute and the water is the solvent.
Explanation:
The salt is the solute.
Question 1 (1 point) Saved Something that tries to disprove its possible explanations is Question 1 options: pseudoscience science non-science Question 2 (1 point) Saved Something that bases conclusions on established authority is Question 2 options: non-science pseudoscience science Question 3 (1 point) Saved In attempting to understand how nature works, scientists seek ultimately for the. Question 3 options: total control of nature most likely explanation final proof truth facts Question 4 (1 point) Something that claims to be scientific, but ignores some of the rules of science is Question 4 options: science non-science pseudoscience Question 5 (1 point) Uncertainty in science makes science unreliable. Question 5 options: True False Question 6 (1 point) Scientific theories. Question 6 options: are heavily supported by evidence begin as hypotheses are scientific guesses attempt to explain and relate large masses of data Question 7 (1 point) Something that often uses supernatural explanations is Question 7 options: non-science pseudoscience science Question 8 (1 point) Any information gained directly or indirectly through our senses is a scientific. Question 8 options: Fact Idea Data Observation Hypothesis Question 9 (1 point) Something that publishes opinions about the most attractive national parks is Question 9 options: science pseudoscience non-science Question 10 (1 point) A hypothesis is best defined as. Question 10 options: a clear statement of a problem a predicted observation the result of an experiment an educated guess a possible solution to a problem. Question 11 (1 point) Those who practice a pseudoscience (e. G. Astrology, biorhythms, etc. ) are those who. Question 11 options: claim to be scientific, yet do not follow all the rules of science attempt to follow the rules of science, but make mistakes in the process do not claim to be scientific and do not follow the rules of science none of these. Question 12 (1 point) In which field(s) is knowledge about how science works important? Question 12 options: Agriculture Politics Law Medicine All of these are correct Question 13 (1 point) When scientists design experiments to test their hypotheses, they are actually trying to ___________ their hypotheses. Question 13 options: Prove Disprove Examine Check Question 14 (1 point) Science can only work with answers that can be tested. Question 14 options: True False Question 15 (1 point) Science can use supernatural explanations if necessary. Question 15 options: True False Question 16 (1 point) Which of these is a limit of science? Question 16 options: It can study the paranormal. Scientific answers never change. It can only answer certain kinds of questions. It can answer any type of question. Question 17 (1 point) Good science is based heavily on the opinions of science experts. Question 17 options: True False Question 18 (1 point) The immediate purpose of a scientific experiment is usually to. Question 18 options: solve a problem create monsters test a hypothesis raise questions. Collect data Question 19 (1 point) A scientific theory is Question 19 options: a general explanation which is highly probable and well supported by testing the same as a scientific law a fact a vague idea Question 20 (1 point) Scientific explanations Question 20 options: can include supernatural explanations cannot include supernatural explanations must include supernatural explanations must show supernatural forces do not exist
Answers
Question 2: something that bases conclusions on established authority is non-science.
Question 3: in attempting to understand how nature works, scientists seek ultimately for the most likely explanation.
Question 4: something that claims to be scientific but ignores some of the rules of science is pseudoscience
Question 5: uncertainty in science makes science unreliable. False.
Question 6: scientific theories attempt to explain and relate large masses of data.
Question 7: something that often uses supernatural explanations is pseudoscience.
Question 8: any information gained directly or indirectly through our senses is a scientific observation.
Question 9: something that publishes opinions about the most attractive national parks is non-science.
Question 10: a hypothesis is best defined as an educated guess.
Question 11: those who practice a pseudoscience claim to be scientific, yet do not follow all the rules of science.
Question 12: knowledge about how science works is important in all of these fields: agriculture, politics, law, and medicine.
Question 13: when scientists design experiments to test their hypotheses, they are actually trying to disprove their hypotheses.
Question 14: science can only work with answers that can be tested. True.
Question 15: science can use supernatural explanations if necessary. False.
Question 16: a limit of science is that it can only answer certain kinds of questions.
Question 17: good science is not based heavily on the opinions of science experts.
False.
Question 18: the immediate purpose of a scientific experiment is usually to collect data.
Question 19: a scientific theory is a general explanation which is highly probable and well supported by testing.
Question 20: scientific explanations cannot include supernatural explanations.
Which statement best describes why most individual organisms never fossilized?(1 point)
Only organisms that lived in the ocean fossilize.
Conditions for fossilization are rare.
Chemicals that fossilize organisms are rare.
Only organisms with hard parts fossilize.
Answers
The statement which best describes why most individual organisms never fossilized is that Conditions for fossilization are rare.
What is fossilization?Fossilization is a term which ia defined as the mechanisms which are chemical, physical, and biological which results in the preservation for long term of animals and plants renants. Fossilization is a type of negative representation of the creatures which is preserved in the substrates as fossil.
Reason for lack of decomposition of fossilizationThe reason for the lack of these fossilized parts can be explained as these parts quickly decompose.
The process of the recycling nutrients which have been consumed by an organism which is used to create its body begins with decomposition.Thus, we concluded that the statement which best describes why most individual organisms never fossilized is that Conditions for fossilization are rare.
learn more about fossilization:
https://brainly.com/question/6867325
#SPJ1
When Mendel crosses a plant with Greenpeace and a plant with yellow peas what color did the offspring have
Answers
Answer:
Yellow Peas
Explanation:
Answer:
yellow
Explanation:
which of the following acids are polyprotic? multiple select question. clch2cooh h3po4 ch4 h2so3
Answers
From the following \(H_3PO_4\) is a polyprotic acid. Polyprotic acids are acids that have more than one ionizable hydrogen atom.
When these acids dissolve in water, they can release multiple protons (H+) in a stepwise manner. In the options you provided: \(H_3PO_4\) (phosphoric acid) is a polyprotic acid because it has three ionizable hydrogen atoms. It can release one proton at a time, forming \(H_2PO4^-\), then \(H_2PO4^-\), and finally \(PO_4^{3-}\). \(H_2SO_3\) (sulfurous acid) is also a polyprotic acid with two ionizable hydrogen atoms. It can release one proton and then release another proton . On the other hand, \(CH_3COOH\) (acetic acid) and \(CH_4\) (methane) are not polyprotic acids. Acetic acid only has one ionizable hydrogen atom and can release one proton to form \(CH_3COO^-\). Methane, which is not an acid, does not have any ionizable hydrogen atoms.
Learn more about polyprotic acid here:
https://brainly.com/question/31116483
#SPJ11
3.50M solution? Show your work!
What is the molarity of these solutions? Show your work!
a. 3.5 mol HBr in 500 mL of water
b. 0.0750 mol NaCl in 250.0 mL of water
c. 1.95 mol AgNO3 in 1.2 L of water
d. 0.500 mol NH3 in 2.5 L of water
Answers
Answer:
a number is correct answer 9
true or false the position of the equlibrium for an endothermic reaction will shift to the right when the reaction mixture is heated explain
Answers
True. the position of the equilibrium of an endothermic reaction will shift to the right when the reaction mixture is heated.
for an endothermic reaction, heat can be treated as a reactant. so when you add more heat the system will shift to get rid of the extra reactant and shift to the right to form more products. Increasing the temperature causes the equilibrium to shift to the right toward a higher concentration of vapor, but, if the system is maintained at that higher temperature, equilibrium will again be established. It is possible to predict how a particular stress or change in conditions will affect an equilibrium. In an increase in temperature will cause the forward reaction to occur, increasing the amounts of the products and decreasing the amounts of reactants. Lowering the temperature will produce the opposite response.
To know more about Endothermic Reaction please visit:
https://brainly.com/question/1160007
#SPJ4
Which item is made from a basic ingredient? (2 points) Soap Tea Wine Vinegar
Answers
Answer:wine
Explanation:it’s made from grapes
A proton and an electron are near each other. What way does their electric force point?
A. No force between them
B. Repulsive
C. Outward
D. Attractive
Answers
Answer:
D
Explanation:
proton is positive while electron is negative so they will attract
The attractive force will be there if a proton and an electron are near each other. Hence, option D is correct.
What is an attractive force?The force by which one object attracts another.
Proton is positive while electron is negative so they will attract.
Hence, the attractive force will be there if a proton and an electron are near each other.
Learn more about attractive force here:
https://brainly.com/question/2396709
#SPJ2
Which of the following best describes what albedo is with respect to earths climate patterns? Why did you pick that answer A) it is the apparent deflection in the forward movement of an object to the right, northward of the equator, and to the left southwest of the equator, due to earth spinning on its axis. B) it is absorption of reflected, radiation from earth surface by atmospheric gases then radiation is then re-emitted, including back to earth surface. C) it is the circulation patterns of global winds outward from the equator or north and south Winds to the altitudes am back.
D) it is the reflection of a proportion of the suns radiation back to the atmosphere by various earth surfaces.
Answers
It is absorption of reflected, radiation from earth surface by atmospheric gases then radiation is then re-emitted, including back to earth surface is the best describes albedo is with respect to earths climate patterns.
What is albedo?
Albedo can be understood as the earth's reflectivity, or the amount of incoming sunlight that is reflected back into space. The main factors that affect global albedo are cloud cover and land-based ice cover, especially in places like Greenland and Antarctica that have substantial ice caps.
What is earth climate ?
The climate of a place refers to its long-term weather patterns. Hour by hour, day by day, month by month, or even year by year, the weather could change. An area's climate is thought to be determined by its weather patterns, which are normally tracked for at least 30 years.
Therefore, It is absorption of reflected, radiation from earth surface by atmospheric gases then radiation is then re-emitted, including back to earth surface is the best describes albedo is with respect to earths climate patterns.
Learn more about albedo from the given link.
https://brainly.com/question/14238698
#SPJ1
copper crystallizes in a face-centered cubic lattice. what is the mass of one unit cell? report your answer in grams. select one: a. 4.22 x 10-22 g b. 2.11 x 10-22 g c. 1.06 x 10-22 g d. 3.17 x 10-22 g
Answers
The mass of one unit copper cell is: A. 4.22 x \(10^{-22}\).
How to calculate the mass of one unit cell?
To determine the mass of one unit cell of copper, which crystallizes in a face-centered cubic lattice, follow these steps:
Find the number of atoms per unit cell. In a face-centered cubic (fcc) lattice, there are 4 atoms per unit cell. So: {(8 corners x 1/8 per corner) + (6 faces x 1/2 per face)}.Determine the molar mass of copper. Copper has an atomic weight of 63.546 g/mol.Calculate the mass of one copper atom. Divide the molar mass of copper by Avogadro's number. So: \(\frac{(63.546 g/mol) }{(6.022 x 10^{-22}atoms/mol) }\) = 1.055 x \(10^{-22}\) g/atom.Calculate the mass of one unit cell. Multiply the mass of one copper atom by the number of atoms per unit cell (4): (1.055 x \(10^{-22}\) g/atom) x 4 atoms = 4.22 x \(10^{-22}\) g.Then, the mass of one unit cell of copper in a face-centered cubic lattice is 4.22 x 10^-22 g. Therefore, he correct answer is A.
Learn more about FCC crystal here https://brainly.com/question/29398453
#SPJ11