Steam is collected and observed to condense into liquid water. Which of the following would you expect to observe if a thermometer recorded the temperature of the substance?
SELECT AN ANSWER
The temperature would decrease.
The temperature would remain the same.
It would depend on the amount of steam.
The temperature would increase.
Answers
the water will be increased
Related Questions
Milk of magnesia, which is an aqueous suspension of magnesium hydroxide, is used as an antacid in the reaction below. How many molecules of HCl would have to be present to form 34.52 g of MgCl₂?
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
Answers
Approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
To determine the number of molecules of HCl required to form 34.52 g of MgCl₂, we need to use the molar mass and stoichiometry of the balanced equation:
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
The molar mass of MgCl₂ is 95.21 g/mol.
First, we need to calculate the number of moles of MgCl₂ formed:
Moles of MgCl₂ = mass of MgCl₂ / molar mass of MgCl₂
Moles of MgCl₂ = 34.52 g / 95.21 g/mol
Moles of MgCl₂ = 0.363 mol
According to the balanced equation, the stoichiometric ratio between HCl and MgCl₂ is 2:1. Therefore, the moles of HCl required can be calculated as follows:
Moles of HCl = 2 * Moles of MgCl₂
Moles of HCl = 2 * 0.363 mol
Moles of HCl = 0.726 mol
To calculate the number of molecules, we need to use Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules of HCl = Moles of HCl * Avogadro's number
Number of molecules of HCl = 0.726 mol * 6.022 x 10^23 molecules/mol
Number of molecules of HCl = 4.37 x 10^23 molecules
Therefore, approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
For more such questions on molecules
https://brainly.com/question/1351818
#SPJ8
The temperature inside my refrigerator is about 40 Celsius. That temperature in Kelvin is K.
I place a balloon in my fridge that initially has a temperature of 220 C. This is K.
If the original volume of the balloon is 0.5 liters, what will be the volume of the balloon when it is fully cooled by my refrigerator? liters. (Round to two decimal places)

Answers
Substituting the given values, we have (0.5 L) / (220 + 273.15 K) = V₂ / (313.15 K).Solving for V₂, we get V₂ = (0.5 L) * (313.15 K) / (220 + 273.15 K).
Calculating this expression, the volume of the balloon when fully cooled by your refrigerator would be approximately 0.38 liters when rounded to two decimal places.To convert Celsius to Kelvin, we need to add 273.15 to the Celsius temperature. Therefore, the temperature inside your refrigerator of 40 degrees Celsius is equivalent to 313.15 Kelvin.Now, let's consider the ideal gas law, which states that PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature in Kelvin.Since the number of moles and pressure remain constant, we can write the equation as V₁/T₁ = V₂/T₂, where V₁ is the initial volume of the balloon, T₁ is the initial temperature, V₂ is the final volume, and T₂ is the final temperature.
for such more questions on values
https://brainly.com/question/27964828
#SPJ8
Which of the following items are made from renewable resources? Select the two correct answers. (1 point)
Responses
plastic fork
plastic fork
metal can
metal can
leather jacket
leather jacket
electronics
electronics
printer paper
Answers
A leather jacket and printer paper are examples of items that can be made from renewable resources, while plastic forks, metal cans, and electronics are not considered renewable due to their reliance on non-renewable materials and processes. Option C, E
The two correct answers that are made from renewable resources are:
C) Leather jacket: Leather is derived from animal hides, which are a byproduct of the meat industry. As long as there is a sustainable and responsible approach to animal farming, the production of leather can be considered renewable. The hides are obtained from animals that are raised for meat consumption, and their use in leather production helps reduce waste.
E) Printer paper: Printer paper can be made from various sources, including trees, bamboo, and recycled paper fibers. If the paper is sourced from sustainably managed forests or from fast-growing plants like bamboo, it can be considered renewable. Additionally, the use of recycled paper fibers reduces the demand for materials and promotes a more circular economy.
The other options, A) plastic fork, B) metal can, and D) electronics, are not made from renewable resources:
A) Plastic fork: Plastics are typically derived from fossil fuels, which are non-renewable resources. The production of plastic involves the extraction and processing of petroleum or natural gas, both of which are finite resources.
B) Metal can: Metal cans are predominantly made from aluminum or steel. While these metals can be recycled, their initial production requires the extraction of raw materials from the Earth, which is not a renewable process.
D) Electronics: Electronics are made from a wide range of materials, including metals, plastics, and various chemical compounds. The production of electronics involves the extraction of raw materials, many of which are non-renewable resources.
Option C and E.
For more such questions on renewable resources visit:
https://brainly.com/question/27734408
#SPJ8
What is the law of multiple proportions?
A. The proportion of elements to compounds is constant.
B. All elements are found in equal proportions in nature.
C. Different compounds may contain the same elements but may have different ratios of those elements.
D. All compounds contain the same elements in the same proportions.
Answers
Answer:
I think
(d) All compounds contain the same elements in the same properties
6. You have 2.3 liters of gas at a pressure of 5.3 atm, and temperature of 45 °C. What will the temperature ofthe gas be if you decrease the volume of gas to 1.2L, and decrease the pressure to 2.5 atm ? 3 pts

Answers
We have a gas that we will assume behaves like an ideal gas. So we can apply the ideal gas law. The ideal gas law tells us:
\(PV=nRT\)Where,
P is the pressure of the gas
V is the volume of the gas
n is the moles of the gas
R is a constant
T is the temperature of the gas
We have two states of the gas. One initial and one final, for both states it is assumed that the moles remain constant. The conditions for each state are.
Initial state:
V1=2.3mL
P1=5.3atm
T1=45°C =318.15K
Final state:
V2=1.2L
P2=2.5atm
T2=?
For each state the ideal gas law will be:
\(\begin{gathered} \frac{P_1V_1}{T_1}=nR \\ \frac{P_2V_2}{T_2}=nR \end{gathered}\)Now, as the moles remain constant, the term nR will be constant and we can equate the two equations, we will then have that:
\(\frac{P_1V_1}{T_1}=\frac{P_2V_2}{T_2}\)Now, we clear T2 and replace the known data:
\(\begin{gathered} \frac{}{}T_2=\frac{P_2V_2}{P_1V_1}\times T_1 \\ T_2=\frac{2.5atm\times1.2L}{5.3atm\times2.3L}\times318.15K \\ T_2=78.30K \end{gathered}\)The temperature of the gas will be 78.3K
Students want to replace the material that connects the battery and the bulb in the circuit. Which material would be the best choice?
A Copper, because it is a conductor
B String, because it is flexible.
C Plastic, because it is an insulator.
D Glass, because light energy can move through it.
Answers
Answer:
a is ok
Explanation:
if they had gold it would be better but no
Write a balanced chemical equation for the standard formation reaction of solid calcium oxide (CaO).
Answers
Answer:
2Ca +O2 ----------> 2CaO ( Balanced Reaction)
The balanced chemical equation for the standard formation reaction of solid calcium oxide (CaO) is
Ca + O2 --> CaO2
Heya are balanced equations?A balanced chemical equation is an equation in which the the number of atoms of each elements on both the reactant and products sides are equal.
Calcium oxide is an alkaline which is solid at room temperature and is formed by the reaction of one atom of calcium and two atoms of oxygen.
Therefore, the balanced equation of formation reaction of solid calcium oxide (CaO) is Ca + O2 --> CaO2.
Learn more about balancing equation here:
https://brainly.com/question/26694427
How could you draw a model of the element copper to show that it is different from the element gold?
Answers
Answer:
You could color it different. Explain in a picture the difference. There are so many different variety's.
Explanation:
For a process Arightwards harpoon over leftwards harpoonB, at 25 °C there is 10% of A at equilibrium while at 75 °C, there is 80% of A at equilibrium. Estimate enthalpy change of this reaction in kJ/mol
Answers
This question is describing the following chemical reaction at equilibrium:
\(A\rightleftharpoons B\)
And provides the relative amounts of both A and B at 25 °C and 75 °C, this means the equilibrium expressions and equilibrium constants can be written as:
\(K_1=\frac{90\%}{10\%}=9\\\\K_2=\frac{20\%}{80\%} =0.25\)
Thus, by recalling the Van't Hoff's equation, we can write:
\(ln(K_2/K_1)=-\frac{\Delta H}{R}(\frac{1}{T_2} -\frac{1}{T_1} )\)
Hence, we solve for the enthalpy change as follows:
\(\Delta H=\frac{-R*ln(K_2/K_1)}{(\frac{1}{T_2} -\frac{1}{T_1} ) }\)
Finally, we plug in the numbers to obtain:
\(\Delta H=\frac{-8.314\frac{J}{mol*K} *ln(0.25/9)}{[\frac{1}{(75+273.15)K} -\frac{1}{(25+273.15)K} ] } \\\\\\\Delta H=4,785.1\frac{J}{mol}\)
Learn more:
https://brainly.com/question/10038290https://brainly.com/question/19671384Which one of the following compounds is insoluble in water?
A) Ba(OH)2
B) Ca3(PO4)2
C) NH4S04
D) Rb2CO3
Answers
Answer:
Ca3(PO4)2
Explanation:
Ca3(PO4)2 or calcium phosphate is insoluble in water.
What type of reaction is this?

Answers
Cu + O2 ---> CuO2 -The first reaction is a combustion reaction
2 HCl + Mg → H2 + MgCl2- The second reaction is a Single replacement reaction
What is a combustion reaction?A combustion reaction is a type of chemical reaction that occurs between a fuel and an oxidizer in the presence of heat or light, resulting in the release of energy in the form of heat and light.
In other words, it is a reaction in which a substance reacts with oxygen to produce heat and light.
Combustion reactions are important in many aspects of daily life, including the burning of fossil fuels for energy production, the combustion of wood or other materials for heating or cooking, and the combustion of fuels in internal combustion engines.
Learn more about combustion reaction:https://brainly.com/question/30562669
#SPJ1
Holt Chemistry Textbook Pg. 014: #01-03
1. Convert each of the following masses to the units requested.
a. 0.765 g to kilograms
b. 1.34 g to milligrams
c. 34.2 mg to grams
d. 23 745 kg to milligrams (Hint: Use two conversion factors.)
2. Convert each of the following lengths to the units requested.
a. 17.3 m to centimeters
b. 2.56 m to kilometers
c. 567 dm to meters
d. 5.13 m to millimeters
3. Which of the following lengths is the shortest, and which is the longest:
1583 cm, 0.0128 km, 17 931 mm, and 14 m?
Answers
The given unit conversions for the mass and length are as follows:
a. 0.765 g = 0.000765 kg
b. 1.34 g = 1340
c. 34.2 mg = 0.0342 g
d. 23 745 kg = 2.3745 * 10⁹ mg
2. a. 17.3 m = 17300 cm
b. 2.56 m = 0.00256 km
c. 567 dm = 56.7 m
d. 5.13 m = 5130 m
3. The shortest length is 0.0128 km and the longest length is 1583 cm
What is the mass of an object?The mass of an object is the amount of matter present in the substance.
The mass of objects is measured using a chemical balance, the unit of measurement is kilograms.
The various units can be interconverted.
To convert each of the following masses to the units requested, the conversion factor is used;
a. 0.765 g to kilograms
0.765 g = 0.765/ 1000
0.765 g = 0.000765 kg
b. 1.34 g to milligrams
1.34 g = 1.34 * 1000
1.34 g = 1340
c. 34.2 mg to grams
34.2 mg = 34.2 / 1000
34.2 mg = 0.0342 g
d. 23 745 kg to milligrams
23 745 kg = 23 745 * 10⁶
23 745 kg = 2.3745 * 10⁹ mg
2. To convert each of the following lengths to the units requested, the steps are as follows:
a. 17.3 m to centimeters
17.3 * 100 cm
17.3 m = 17300 cm
b. 2.56 m to kilometers
2.56 m = 2.56/ 1000
2.56 m = 0.00256 km
c. 567 dm to meters
567 dm = 567 / 10
567 dm = 56.7 m
d. 5.13 m to millimeters
5.13 m = 5.13 * 1000
5.13 m = 5130 m
3. Converting the following lengths to meters:
1583 cm = 15.83 m
0.0128 km = 12.8 m
17 931 mm = 17.931 m
14 m
The shortest length is 0.0128 km and the longest length is 1583 cm
Learn more about length and mass at: https://brainly.com/question/18270472
#SPJ1
What compound has 4 hydrogen atoms and one carbon
Answers
Carbon atoms may thus form bonds to as many as four other atoms. For example, in methane (CH 4start subscript, 4, end subscript), carbon forms covalent bonds with four hydrogen atoms
__________________________________________________________
What is the percent composition of Fluorine (F) in the compound XeF6?
Od
26.258%
12.520%
110.76%
46.472%
Answers
The percent by mass of the fluorine in the compound is 46.472%.
What is the percent by mass?We know that the percent by mass has to do with the ratio of the total mass of the atom that is part of the compound and the total molar mass of the compound multiplied by one hundred.
The question in this case has demanded that we ought to obtain the mass percent of fluorine from the compound that we can be able to identify from the formula of the compound that is shown here as xenon hexa fluoride.
Mass of the compound can be obtained from; 131 + 6(19)
= 245 g/mol
The total mass of the fluorine atom in the compound is 114 g
Thus we have the use of; 114 /245 * 100/1
= 46.472%
The percent by mass is now gotten for the fluorine atom as 46.472%.
Learn more about percent by mass:https://brainly.com/question/5394922
#SPJ1
how many sugar molecules are present in 300mL of a 2.0 m solution
Answers
The amount of sugar molecules present in 300mL of a 2.0M solution is 3.61 × 10²³ molecules of sugar.
How to calculate number of molecules?The number of molecules of a substance can be calculated by multiplying the number of moles in the substance by Avogadro's number (6.02 × 10²³).
According to this question, 300mL of a 2.0M solution is given. The molarity of the solution can be calculated as follows:
no of moles = 0.300 × 2.0 = 0.6moles
no of molecules = 0.6mol × 6.02 × 10²³
no of molecules = 3.61 × 10²³ molecules of sugar.
Learn more about no of molecules at: https://brainly.com/question/28931982
#SPJ1
The student's lab manual says to mix some of his Na2CO3 solution with an aqueous solution of copper(II) sulfate (CuSO4)
i What evidence of a chemical reaction would he expect to see? Explain your answer.
ii Write a balanced chemical equation to show the reaction. Include state symbols.
iii What kind of reaction is this?
Answers
i When sodium carbonate (Na2CO3) is mixed with an aqueous solution of copper(II) sulfate (CuSO4), the student can expect to see several evidence of a chemical reaction:
Formation of a solid precipitate: When these two solutions are mixed, a solid precipitate of copper(II) carbonate (CuCO3) will form. This is a sign that a chemical reaction has occurred.
Change in color: The reaction between sodium carbonate and copper(II) sulfate will also result in a change in color. The solution may turn a blue or green color, indicating the presence of copper(II) ions.
Release of gases: The reaction between sodium carbonate and copper(II) sulfate may also produce gases, such as carbon dioxide (CO2).
ii The balanced chemical equation for the reaction between sodium carbonate and copper(II) sulfate is:
2Na2CO3(aq) + CuSO4(aq) → 2Na2SO4(aq) + CuCO3(s)
iii This is a double displacement reaction, also known as a metathesis reaction. In this type of reaction, the cations (positively charged ions) and anions (negatively charged ions) of the reactant compounds exchange places to form the products. In this case, the sodium ions (Na+) and the copper ions (Cu2+) exchange places to form sodium sulfate (Na2SO4) and copper carbonate (CuCO3).
can you Explain the process of how clouds form?????
Answers
Answer: Clouds need two things to form: cool air and particles on which water vapor can condense. Clouds form as air cools. Cold air cannot hold as much water vapor, so the water vapor condenses. Water vapor condenses on small particles in the atmosphere, such as smoke, salt, and dust. As a result, clouds form.
Explanation: correct on edge
A solution is made by dissolving 38.81 grams of nickel (II) sulfate, NiSO4, in enough water to make 0.467
liters of solution. Calculate the molarity of this solution.
Answers
The molarity of the NiSO₄ solution made by dissolving 38.81 grams of nickel (ii) sulfate, NiSO₄, in enough water to make 0.467 liters of solution is 0.535 M
How do i determine the molarity of the solution?First, we shall obtain the mole of 38.81 grams of nickel (ii) sulfate, NiSO₄. Details below:
Mass of NiSO₄ = 38.81 grams Molar mass of NiSO₄ = 154.75 g/molMole of NiSO₄ = ?Mole of NiSO₄ = mass / molar mass
= 38.81 / 154.75
= 0.25 mole
Now, we shall determine the molarity of the solution. Details below:
Mole of NiSO₄ = 0.25 moleVolume of solution = 10.467 LMolarity of solution = ?Molarity of solution = mole / volume
= 0.25 / 0.467
= 0.535 M
Thus, the molarity of the solution is 0.535 M
Learn more about molarity:
https://brainly.com/question/16073358
#SPJ1
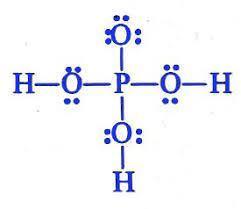
Which is the Lewis structure for H3PO4? An upper P is single bonded above to an O, and to the left, right, and below to an O single bonded to an H. The O above the P has three pairs of dots to the left, above, and below; the O's to the sides have pairs of dots above and below, and the O below the P has pairs of dots right and left. A central upper P is single bonded left, right, above, and below to upper Os. The O above the P is single bonded to upper H on the left and the right, and has two electron dots above it. The O below the P is single bonded to an H below, and has pairs of electron dots to the left and right. A central upper P is double bonded to an O above, and single-bonded to an upper O single-bonded to an upper H to the left and the right. The O above the P has three pairs of electron dots, to the left, above, and to the right; the O's to the right and left have pairs of dots above and below. A central upper P is bonded to an upper H above, an upper O below, and upper O's bonded to upper H's to the left and the right. The O below the P has three pairs of electron dots, to the left, right, and below; the other two O's have pairs of dots above and below. A central upper P is double bonded to an O above, and single-bonded to an upper O single-bonded to an upper H to the left and the right. The O above the P has three pairs of electron dots, to the left, above, and to the right; the O's to the right and left have pairs of dots above and below.
Answers
Answer:
It is A.
Explanation:
I took the test.
The Lewis structure shows the arrangement of valence electrons in H3PO4.
The Lewis structure gives us a picture of the number of valence electrons in a molecule. This is because, in a Lewis structure, the electrons in the molecule are shown as dots. A single line may be used to show shared electrons in a covalent bond.
The correct Lewis structure of H3PO4 is an upper P is single bonded above to an O, and to the left, right, and below to an O single bonded to an H. The O above the P has three pairs of dots to the left, above, and below; the O's to the sides have pairs of dots above and below, and the O below the P has pairs of dots right and left.
Learn more: https://brainly.com/question/4144781
How could you tell if a substance has undergone a physical change or a chemical one?
Answers
Answer: Chemical changes occur when a substance combines with another to form a new substance, called chemical synthesis or, alternatively, chemical decomposition into two or more different substances. These processes are called chemical reactions and, in general, are not reversible except by further chemical reactions.
A physical change is are changes affecting the form of a chemical substance, but not its chemical composition. Physical changes are used to separate mixtures into their component compounds, but can not usually be used to separate compounds into chemical elements or simpler compounds.
For this same exercise, if 2.5 liters of this solution (2,500 ml) are administered in a 24-hour interval, what amount in grams of glucose did the patient receive in one day? the glucose to dissolve is 50 grams
Answers
Answer:
j
Explanation:
becaisdbyhe wrl is so not goood
What specialized plant structures increase the probability of successful reproduction?
Answers
Answer:
The pistil and the stamen.
Explanation:
Please Mark Me Branliest.
The pistil and the stamen are the specialized structures increase the probability of successful plant reproduction
what is reproduction in plants ?Reproduction in Flowering Plants occur both asexually and sexually, flowering plants or angiosperms, use the sexual mode of reproduction.
Reproduction in Flowering Plants occurs in the flower as both male and the female gametes are present in it, various parts of flower involve in the process of reproduction.
There are four important steps in the reproduction of flowering plants. such as Pollination, Gametogenesis, Fertilization
The flower is a specialized structure where reproductive part of the plant, containing the male and female reproductive organs which facilitate fertilization.
For more details regarding plant reproduction, visit
brainly.com/question/6750187
#SPJ6
A 0.042 mol sample of Ca(OH)2 requires 51.21 mL of aqueous HCl for neutralization. What is the concentration of the HCl
Answers
Answer:
Conc = 1.64 Mol / dm3
Explanation:
We have to first put down the equation of the neutralization reaction. This is given as;
2HCl + Ca(OH)2 --> CaCl2 + 2H2O
This means that 2 mol of HCl requires 1 mol of Ca(OH)2.
2 = 1
x = 0.042
x = 0.084 mol of HCl required.
Volume = 51.21 ml = 0.05121 dm3
Concentration = Mass / Volume
Conc = 0.084 / 0.05121
Conc = 1.64 Mol / dm3
how many electrons are in an atom with the electron configuration of 1s22s22p63s1
Answers
Answer:
11
Explanation:
From the question given above, the following data were obtained:
Electronic configuration => 1s²2s²2p⁶3s¹
Number of electrons =?
We must understand that the electronic configuration of an element is written based the number of electrons present in the atom of the element.
To obtain the number of electrons in the atom given in the question above, we simply add up the electrons in each orbital. This can be obtained as follow:
Electronic configuration => 1s²2s²2p⁶3s¹
Number of electrons =?
Number of electrons = 2 + 2 + 6 + 1
Number of electrons = 11
Thus , the number of electrons in the atom is 11
Helium is a....
a. metalloid
b. nonmetal
c. metal
Answers
Answer:
NonmetalExplanation:
even one or two crystals of copper sulphate can make its solution in water coloured blue. why
Answers
this also may happen because of the water molecules that get attached
Which of these statements is supported by the results of Thomson’s experiment? Check all of the boxes that apply
Answers
Answer:
1) Cathode rays are made up of negatively charged particles. 2)atoms contain negatively charged particles.3)Thomson's results led to the proposal of new atomic model the plum pudding model.
Explanation:
Answer:
B. Cathode rays are made up of negatively charged particles
C. Atoms contain negatively charged particles
Explanation:
Edge 2021
Endocrine glands release chemicals that are also known as ___. a. target cells. b. hypothalamus. c. lymph. d. hormones. Please select the best answer from the choices provided A B C D
Answers
Answer:
Endocrine glands release chemicals that are also known as hormones.
Explanation:
Hope this helped!!!
Answer:
D. Hormones
Explanation:
The Endocrine system deals with all things hormones such as growth development and anything else dealing with puberty
What is the value of secant theta given the diagram below?
Answers
Answer:
The value of sec theta is
How to determine the value of sec theta
From the diagram, we start by calculating the length of the hypotenuse (h).
So, we have:
Evaluate
Simplify
The value of the secant in the second quadrant is calculated as:
So, we have:
Evaluate
Hence, the value of sec theta is
Read more about trigonometry at:
brainly.com/question/11967894
THANKS
11
5.0
(6 votes)
Explanation:
hope it helps
Why is knowing the concentration of solutions important in the real world? Give an example to help you explain your answer.
Answers
Explanation: The concentration of a solution helps us to determine the collision speed between particles in a molecule or compound. Knowing the concentrations of components in solutions can help determine the health of the world.