Answers
Related Questions
hexaphosphorus nonasulfide formula
Answers
Answer:
P6S9
Explanation:
Firstly, let's write the numbers in Latin
1 = mono
2 = di
3 = tri
4 = tetra
5 = penta
6 = hexa
7 = hepta
8 = octa
9 = nona
Secondly, write the symboles of the given elements:
Phosphorus is P
Sulfide is S
Finally, connect the numbers and symbols.
Rule of pronunciation: Number of first element + symbol of first element + number of second element + symbol of second element
P6S9
Please upvote.
2 FeCl2
+ 13 KClO4
+ H2
SO4
→ 2 Fe(ClO3
)3
+
K2
SO4
+ 11 KClO3
+ H2
O
Calculate the grams of KClO3
arising from the
reaction of 2.50 g of KClO4
with 150 mg of FeCl2
in excess of H2
SO4
.
Answers
Taking into account the reaction stoichiometry and limiting reagent, 0.7977 grams of KClO₃ are formed from the reaction of 2.50 g of KClO₄ with 150 mg of FeCl₂ in excess of H₂SO₄.
In first place, the balanced reaction is:
2 FeCl₂ + 13 KClO₄ + H₂SO₄ → 2 Fe(ClO₃)₃ + K₂SO₄ + 11 KClO₃ + H₂O
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
FeCl₂: 2 molesKClO₄: 13 moles H₂SO₄: 1 moleFe(ClO₃)₃: 2 moles K₂SO₄: 1 mole KClO₃: 11 moles H₂O: 1 moleThe molar mass of the compounds is:
FeCl₂: 126.75 g/moleKClO₄: 138.55 g/mole H₂SO₄: 98 g/moleFe(ClO₃)₃: 306.2 g/moleK₂SO₄: 174.2 g/moleKClO₃: 122.55 g/moleH₂O: 18 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
FeCl₂: 2 moles× 126.75 g/mole= 253.5 gramsKClO₄: 13 moles× 138.55 g/mol= 1801.15 grams H₂SO₄: 1 mole× 98 g/mole= 98 gramsFe(ClO₃)₃: 2 moles× 306.2 g/mole= 612.4 gramsK₂SO₄: 1 mole× 174.2 g/mole= 174.2 gramsKClO₃: 11 moles× 122.55 g/mole= 1348.05 gramsH₂O: 1 mole× 18 g/mole= 18 grams
The limiting reagent is one that is consumed first in its entirety, determining the amount of product in the reaction. When the limiting reagent is finished, the chemical reaction will stop.
To determine the limiting reagent, it is possible to use the reaction stoichiometry of the reaction and a simple rule of three as follows: if by stoichiometry 1801.15 grams of KClO₄ reacts with 253.5 grams of FeCl₂, if 2.50 grams of KClO₄ react how many mass of FeCl₂ will be needed?
\(mass of FeCl_{2}=\frac{2.50 grams of KClO_{4} x253.5 grams of FeCl_{2}}{1801.15 grams grams of KClO_{4}}\)
mass of FeCl₂=0.35 grams
But 0.35 grams of FeCl₂ are not available, 150 mg= 0.150 grams (being 1000 mg= 1 grams) are available. Since you have less mass than you need to react with 1801.15 grams of KClO₄, FeCl₂ will be the limiting reagent.
Then the following rule of three can be applied: if by reaction stoichiometry 253.5 grams of FeCl₂ form 1348.05 grams of KClO₃, 0.150 grams of FeCl₂ form how much mass of KClO₃?
\(mass of KClO_{3}=\frac{0.150 grams of FeCl_{2}x1348.05 grams of KClO_{3} }{253.5grams of FeCl_{2} }\)
mass of KClO₃= 0.7977 grams
Then, 0.7977 grams of KClO₃ are formed from the reaction of 2.50 g of KClO₄ with 150 mg of FeCl₂ in excess of H₂SO₄.
Learn more about reaction stoichiometry:
brainly.com/question/24741074 brainly.com/question/24653699 brainly.com/question/23871710A 1.250-g sample of benzoic acid, C7H6O2, was placed in a combustion bomb. The bomb was filled with an excess of oxygen at high pressure, sealed, and immersed in a pail of water which served as a calorimeter. The heat capacity of the entire apparatus (bomb, pail, thermometer, and water) was found to be 10.134 kJ/K. The oxidation of the benzoic acid was triggered by passing an electric spark through the sample. After complete combustion, the thermometer immersed in the water registered a temperature 3.256 K greater than before the combustion. What is DEcombustion per mole of benzoic acid burned
Answers
Answer:
3224 kJ/mol
Explanation:
The combustion of benzoic acid occurs as follows:
C₇H₆O₂ + 13/2O₂ → 7CO₂ + 3H₂O + dE
The change in temperature in the reaction is the change due the energy released, that is:
3.256K * (10.134kJ / K) = 33.00kJ are released when 1.250g reacts
To find the heat released per mole we have to find the moles of benzoic acid:
Moles benzoic acid -Molar mass: 122.12g/mol-:
1.250g * (1mol / 122.12g) = 0.0102 moles
The dE combustion per mole of benzoic acid is:
33.00kJ / 0.0102moles =
3224 kJ/mol
Explain collision theory in your own words and then apply this collision to explain the effect of surface area on reaction rates. Draw a graph illustrating how surface area of reactants affects reaction rate. Please be sure to label all parts of the graph.
Answers
Collision theory. Molecules must collide with each other for the reaction to occur. The orientation also plays an important role since molecules must collide in a specific orientation. Also, molecules must have enough energy to react.
Surface area. When the area of a solid is increased, the rate of the reaction increases. This is because more particles are exposed to react.

A 85.4 lb child has a Streptococcus infection. Amoxicillin is prescribed at a dosage of 45 mg per kg of body weight per day given b.i.d.
Answers
The amount of Amoxicillin dose given to the 85.4 lb child daily is determined as 1,743.3 mg.
What is the amount of Amoxicillin dose given to the child?
The amount of Amoxicillin dose given to the child is calculated as follows;
amount of Amoxicillin dose = weight of the child x dosage prescribed
What is the weight of the child in pounds (lb)The weight of the child in pounds (lb) is calculated as follows;
1 lb = 0.453592 kg
85.4 lb = ?
= 85.4 x 0.453592 kg
= 38.74 kg
amount of Amoxicillin dose = 38.74 kg x 45 mg/kg
amount of Amoxicillin dose = 1,743.3 mg
Thus, the amount of Amoxicillin dose given to the 85.4 lb child daily is determined as 1,743.3 mg.
Learn more about amount of dose here: https://brainly.com/question/11185154
#SPJ1
The complete question is below:
A 85.4 lb child has a Streptococcus infection. Amoxicillin is prescribed at a dosage of 45 mg per kg of body weight per day given b.i.d. Calculate the daily dose of the child.
Using the reading "Fossil Fuels" from lesson 12 describe the environmental and economic benefits and drawbacks of fossil fuels. Second, looking over the benefits and drawbacks, in your opinion, what do you think will happen to mining of fossil fuels in the next 50 years?
Answers
Fossil fuels are essential part of the power generation in the world. They are easily combustible and more reliable and cheaper. However, the burning of fossil fuels releases toxic gases to the environment.
What are fossil fuels ?Fossil fuels are fuel generated from the decomposition materials. Petroleum, coal, natural gas etc. are fossil fuels which are excavating from the earth.
Fossil fuels are non-renewable sources of energy. Hence, as the existing fossil sources are exhausted no more fossil fuel can be made. It is cheaper, reliable and easy to use.
However, the toxic hydrocarbon gases released from the burning of fossil fuels make the environment polluted. Therefore, overuse of fossil fuel definitely rise the atmospheric pollution.
Its use over the next 50 years, will increase the global warming and more of it will be exhausted.
Find more on fossil fuels :
https://brainly.com/question/3371055
#SPJ1
How many moles of O2, form as 80 g of KClO3 are totally consumed?
M(KCIO3)= 122.45 g/mol.
Answers
Answer:
0.975 mole of O₂
Explanation:
We'll begin by calculating the number of mole in 80 g of KClO₃. This can be obtained as follow:
Molar mass of KClO₃ = 122.45 g/mol.
Mass of KClO₃ = 80 g
Mole of KClO₃ =?
Mole = mass /Molar mass
Mole of KClO₃ = 80 / 122.45
Mole of KClO₃ = 0.65 mole
Next, we shall write the balanced equation for the reaction. This is illustrated below:
2KClO₃ —> 2KCl + 3O₂
From the balanced equation above,
2 moles of KClO₃ decomposed to produce 3 moles of O₂.
Finally, we shall determine the number of mole O₂ produced by the decomposition of 80 g (i.e 0.65 mole) of KClO₃. This can be obtained as follow:
From the balanced equation above,
2 moles of KClO₃ decomposed to produce 3 moles of O₂.
Therefore, 0.65 mole of KClO₃ will decompose to produce = (0.65 × 3)/2 = 0.975 mole of O₂.
Thus, 0.975 mole of O₂ was obtained from the reaction.
CAN SOMEONE HELP WITH THIS QUESTION?

Answers
1a. The theoretical percentage of water in nickel(II) sulfate heptahydrate is 44.9%
1b. The theoretical percentage of water in aluminum chloride hexahydrate is 44.7%
1a. How do I determine the percentage of water?The percentage of water in nickel(II) sulfate heptahydrate, NiSO₄.7H₂O can be obtained as follow:
Molar mass of NiSO₄.7H₂O = 280.7 g/moleMass of H₂O in NiSO₄.7H₂O = 7H₂O = 7 × 18 = 126 gPercentage of water, H₂O =?Percentage of water, H₂O = (mass of H₂O / mass of NiSO₄.7H₂O) × 100
Percentage of water, H₂O = (126 / 280.7) × 100
Percentage of water, H₂O = 44.9%
1b. How do I determine the percentage of water?The percentage of water in aluminum chloride hexahydrate, AlCl₃.6H₂O can be obtained as follow:
Molar mass of AlCl₃.6H₂O = 241.5 g/moleMass of H₂O in AlCl₃.6H₂O = 6H₂O = 6 × 18 = 108 gPercentage of water, H₂O =?Percentage of water, H₂O = (mass of H₂O / mass of AlCl₃.6H₂O) × 100
Percentage of water, H₂O = (108 / 241.5) × 100
Percentage of water, H₂O = 44.7%
Learn more about percentage composition:
https://brainly.com/question/11952337
#SPJ1
Select the best answer for the question. 1. Mei is seated doing leg extensions and going through the full path of motion. What type of exercise is Mei doing? O A. Free-weight exercise B. Resistance exercise C. Machine exercise O D. Cable exercise
Answers
The correct answer is "C.
The type of exercise that Mei is doing is the "Machine exercise."
Machine exercise refers to a physical fitness training technique that allows the muscles to develop and strength through the use of machines that use hydraulic cylinders, weights, and cables to produce resistance. The machine exercises are generally performed in a seated position or lying down, and most often use a series of cables and weights that are adjusted to the user's specific body weight and desired level of resistance.Machine exercises can effectively target specific muscle groups and help strengthen them.Machine exercises can help you increase muscular endurance and improve your overall fitness level.Machine exercises are often safer and easier to perform than free-weight exercises.Machine exercises are generally easier on your joints and can help reduce the risk of injury.Machine exercises are also helpful for people with limited mobility or those recovering from an injury or surgery.For such more questions on Machine exercise
https://brainly.com/question/29402478
#SPJ8
The total number of sodium atoms in 46.0 grams of sodium
is
Answers
HELP ASAP PLEASE ANSWER BOTH OF THESE QUESTIONS! I WILL GIVE YOU BRAINELIST IF YOU ANSWER IT!


Answers
Answer:
because it absorbs all colours expect for red,
Explanation: making the red light bounce into your eyes, therefore seeing the colour red
Question 2: The wave transfers its energy to the mineral, I think, thats my best guess
Acetylene (C₂H₂) gas is often used in welding torches because of the very high heat produced when it reacts with oxygen (O₂) gas, producing carbon dioxide
gas and water vapor. Calculate the moles of water produced by the reaction of 2.2 mol of acetylene. Be sure your answer has a unit symbol, if necessary, and
round it to the correct number of significant figures
Answers
Explanation:
To calculate the moles of water produced, we first need to write down the balanced chemical equation for the combustion of acetylene:
C₂H₂(g) + O₂(g) → CO₂(g) + H₂O(g)
To balance the equation, we need to make sure that there are the same number of atoms for each element on both sides of the equation. The balanced equation is:
2C₂H₂(g) + 5O₂(g) → 4CO₂(g) + 2H₂O(g)
Now we can use stoichiometry to find the moles of water produced:
2.2 mol C₂H₂ * (2 mol H₂O / 2 mol C₂H₂) = 2.2 mol H₂O
So, 2.2 moles of acetylene will produce 2.2 moles of water when reacted with a sufficient amount of oxygen.
What are you allowed to do to balance a chemical equation?
Answers
On a mission to a newly discovered planet, an astronaut finds copper abundances of 69.15% for "Cu and 30.85 % for Cu. What is the atomic mass of copper for this location? What are the units
Answers
The units for atomic mass are atomic mass units (amu). the atomic mass of copper for this location is 63.55 amu.
The chemical symbol Cu stands for copper. Copper is a soft, malleable, ductile metal that is a good conductor of heat and electricity. Copper is one of the most widely used metals in electrical and electronic equipment due to its superior conductivity and non-corrosive properties. This metal is widely used in wiring, roofing, plumbing, and electronic applications. Its atomic mass is 63.55 amu.The atomic mass of copper for this location can be determined using the following formula:
Atomic mass = (mass of isotope 1 x relative abundance of isotope 1) + (mass of isotope 2 x relative abundance of isotope 2)The atomic mass of copper for this location
= (62.93 x 0.6915) + (64.93 x 0.3085) = 63.55 amu
For more question atomic mass
https://brainly.com/question/30402000
#SPJ8
PLEASE HELPPPP ASAPPPPPP!!!!!!!

Answers
Answer:
that the oxygen atom is not equal on both sides
Explanation:
Thus the reaction is not balanced.
A cleaning product company is having trouble with the phi control of one of their products. The prod basic but the pH is too high What could the company try to correct the pH of the product? A. Increasing the amount of product in each container B. Diluting the contents of each container by adding water C. Allowing some water to evaporate from each container D. Adding drain cleaner from this activity to each container (tts)
Answers
Answer:
Option B.
Explanation:
Obviously, a cleaning agent companies is going to be a producer of cleaning agents which are chemical substance which are used for the main purpose of removing unwanted particles (dirt).
For this particular Question, option ''B'' is correct because upon the addition of H2O, there will surely be changes in the numbers of ions in the cleaning agents.
From the Question above, we have that the cleaning agents has pH which is too high, therefore in order to reduce the pH of the cleaning agents, water will be used to mix the product or cleaning agents. Upon the addition of water there will be decrease in OH^-.
Kepler's third law of planetary motion describes the relationship between the
of a planet's orbit and its period,
A. major axis
B. semi-
major axis
C. acceleration
D. velocity
Answers
Answer:
B. semi-major axis is the answer, I hope that helps
How many moles of carbon atoms do you have if you have 48.4 g of carbon?
Answers
Answer:
4.03 moles
Explanation:
We know that the molar mass of Carbon is 12.011, thus we can solve for moles by dividing the grams given by the molar mass.
48.4 g / 12.011 g = 4.03 moles
Which type of reaction is NaCl + AgNO3 → NaNO3 + AgCl?
synthesis
decomposition
oxidation
replacement
Answers
Answer:
replacement
Explanation:
What is another example, in real life, where we can prove that gases exist even though we can not see them? Explain why you believe this is a good example.
Answers
Well, us human being rely on \(o_{2}\) (oxygen). We human beings breathe this in every day because we need it to survive. This is a good example because it explains how humans don't see \(o_{2}\) but use it every day.
which 2 criteria are the most important of engineers to consider when developing a procsses to produce
Answers
Two key criteria that engineers must prioritize are efficiency and safety. By emphasizing efficiency and safety during process development, engineers can create robust and reliable processes that not only maximize productivity but also prioritize the well-being of personnel and the environment.
When developing a process, engineers need to consider several important criteria. Two key criteria that engineers must prioritize are efficiency and safety.
Efficiency is crucial in process development to ensure optimal use of resources, time, and energy. Engineers strive to design processes that maximize productivity, minimize waste, and reduce costs. This involves optimizing reaction conditions, streamlining workflow, and implementing automation where possible. Efficiency considerations also extend to energy consumption, raw material utilization, and overall process sustainability.
Safety is another critical aspect that engineers must prioritize. They need to identify and mitigate potential hazards associated with the process, ensuring the safety of both personnel and the environment. This involves conducting thorough risk assessments, implementing safety protocols, and designing equipment and systems with safety features. Engineers must also consider the safe handling and storage of materials, as well as potential risks during transportation and disposal.
By emphasizing efficiency and safety during process development, engineers can create robust and reliable processes that not only maximize productivity but also prioritize the well-being of personnel and the environment.
For more question on environment
https://brainly.com/question/1186120
#SPJ8
Which is one use for infrared waves?
A)To provide heat for pets or livestock
B)To power nuclear weapons
C)To operate a machine that cooks food quickly
D)To light desk lamps
Answers
A contraption that swiftly prepares food is run by infrared rays. Microwaves employ infrared rays to heat meals by warming any water that may be present.
What are some uses for infrared and microwave technology?Numerous food production processes, including drying, boiling, heating, peeling, polyphenol recovery, freeze-drying, antioxidant recovery, microbiological inhibition, sterilizing grains, baking bread, roasting food, making juices, and cooking food, all use infrared technology.
What are some uses and applications for infrared waves?Infrared radiation has the ability to ease or release muscle tension and encourage local blood circulation in the body. Infrared radiation has been used in conventional and modern medicine to treat conditions including autoimmune diseases and issues with wound healing in addition to relieving muscle pain and tension.
To learn more about infrared waves visit:
brainly.com/question/30309627
#SPJ1
what is the change in mass of A in
60 minutes?
Mass of A (g)
12.4
10.4
9.1
7.7
6.2
Time
O
15
30
45
60
Answers
Answer:
To determine the change in mass of A over the given time period, we need to find the difference between the initial mass of A and the final mass of A.
From the given table, we can see that the initial mass of A at t = 0 (start time) is 12.4 g and the final mass of A at t = 60 minutes (end time) is 6.2 g.
Therefore, the change in mass of A over 60 minutes is:
Final mass of A - Initial mass of A
= 6.2 g - 12.4 g
= -6.2 g
The negative sign indicates that the mass of A decreased over time, which means that A underwent some kind of reaction or process that caused it to lose mass.
The change in mass of A over 60 minutes is -6.2 grams.
To determine the change in mass of A over 60 minutes, we need to compare the initial mass to the final mass.
From the given information, we can see that the mass of A decreases over time.
Let's calculate the change in mass.
Initial Mass of A: 12.4 g
Final Mass of A: 6.2 g
Change in Mass of A = Final Mass of A - Initial Mass of A
= 6.2 g - 12.4 g
= -6.2 g
The change in mass of A over 60 minutes is -6.2 grams.
Note that the negative sign indicates a decrease in mass.
For such more questions on mass
https://brainly.com/question/1838164
#SPJ8
How much heat is required to raise the temperature of 1,500 g of water from 25°C to 52°C? The specific heat of water is 4.184 J/g-oC.
Answers
Apply thermodynamics
\(\\ \rm\rightarrowtail Q=mc\delta T\)
Q is heat\(\\ \rm\rightarrowtail Q=1500(4.184)(27)\)
\(\\ \rm\rightarrowtail Q=169452J\)
a person uses a force of 27 n to hold a stationary trash can a distance of .5 above the ground. how much work is done on the garbage can?
Answers
Answer:
135
Explanation:
Work can be calculated with the equation: Work = Force × Distance
Hope it helps!!!Brainliest pls!!!A marble has a density of 11 g/cm3 and when dropped in a graduated cylinder increases the water level from 2 mL to 4 mL of volume. What is the mass of the marble in grams?
Answers
Answer:
The answer is 22 gExplanation:
The mass of a substance when given the density and volume can be found by using the formula
mass = Density × volumeFrom the question
density = 11 g/cm³
volume = final volume of water - initial volume of water
volume = 4 - 2 = 2 mL
But 1 mL = 1 cm³
2 mL = 2 cm³
volume = 2 cm³
The mass of the marble is
mass = 11 × 2
We have the final answer as
22 gHope this helps you
state why vulcanologist make predications about when a volcano will erupt
Answers
Answer:
They want to know how long it has been since it last erupted.
Explanation:
Scientists study a volcano's history to try to predict when it will next erupt. They also want to know the time span between its previous eruptions
If the student’s estimate of the balloon’s volume was incorrect and the actual volume was 620 ml, would the amount of glucose that actually reacted be more than or less than the amount calculated in part (c)? Explain your response.
( C answer ) only 1.9 g of glucose reacted and only .0211 mol of co2 was formed.

Answers
The number of moles of CO2 produced is 0.021 moles
If the estimated volume of the balloon is wrong then the amount of glucose reacted must be more than is stated.
What is respiration equation?The respiration equation represents the chemical process of aerobic cellular respiration, which occurs in the mitochondria of cells and is the primary way in which cells generate energy in the form of ATP (adenosine triphosphate).
The equation of the reaction is;
C6H12O6 + 6O2 → 6CO2 + 6H2O + ATP
We know that;
Number of moles of glucose = 10 g/180 g/mol
= 0.056 moles
PV = nRT
n = PV/RT
n = 1 * 0.55/318 * 0.082
n = 0.021
Learn more about glucose:https://brainly.com/question/2252123
#SPJ1
Which of the following describes green design?
A. Materials that will fail in a predictable and safe way
B. The use of new engineering technologies in building construction
c. Building structures made of composite materials to make them
safer
D. Materials and design techniques that reduce the negative
environmental impact of a structure
Answers
Answer: materials and design Techniques that reduce the negative environmental impact of a structure
Explanation:
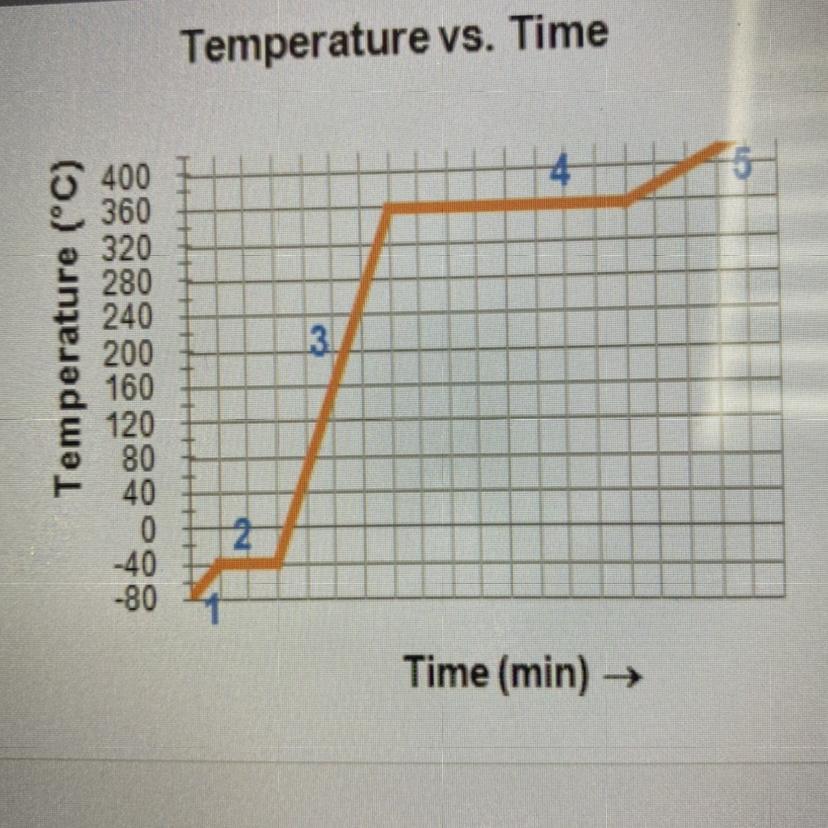
Complete the statements by writing the number
from the graph.
The substance is in the gas phase only in region
The substance is in both the liquid and the solid
phase in region
The substance is in only the liquid phase in region
The melting point is the temperature at region
The boiling point is the temperature at region
Answers
Answer :
The substance is in the gas phase only in region → 5
The substance is in both the liquid and the solid phase in region → 2
The substance is in only the liquid phase in region → 3
The melting point is the temperature at region → 2
The boiling point is the temperature at region → 4
Explanation :
Six phases of substance:
Melting or fusion : In this process the phase changes from solid state to liquid state at constant temperature.Freezing : In this process the phase changes from liquid state to solid state at constant temperature.Evaporation : In this process the phase changes from liquid state to gaseous state at constant temperature.Condensation : In this process the phase changes from gaseous state to liquid state at constant temperature.Sublimation : In this process the phase changes from solid state to gaseous state without passing through the liquid state at constant temperature.Deposition : In this process the phase changes from gaseous state to solid state without passing through the liquid state at constant temperature.Answer:
5, 2, 3, 2, 4.
Explanation:
got it correct on edge 2021