Solid calcium phosphate and aqueous sulfuric acid solution react to give calcium sulfate, which comes out of solution as a solid. The other product is phosphoric acid, which remains in solution. Write an equilibrium equation for the reaction using complete formulas for the compounds with phase labels.
Answers
Answer: i also need help on the same question :(
Explanation:
Related Questions
How many moles of a gas are in a 2.2-liter balloon at 1.3 atm and 85 C. Also asks to identify the P, V, N, R, and T. If unknown then put "?" HELP!!
Answers
Answer:
n= 0.059 moles of gas
Explanation:
Potassium has a larger ionic radius than sodium. Explain which of KCl and NaCl has stronger bonding. (2 marks)
Answers
STEP-BY-STEP EXPLANATION:
Firstly, we need to define the ionic radius
The ionic radius is the distance between the nucleus and the electron in the outermost shell of an ion.
Recall, that the ionic radius decreases across the period and increases down the group
Given that the atomic number of Potassium is 19 and the atomic number of sodium is 11
The next thing is to write the electronic configuration of both elements
\(\begin{gathered} _{19}K=1s^22s^22p^63s^23p^64s^1 \\ _{11}Na=1s^22s^22p^63s^1 \end{gathered}\)According to the electronic configuration of sodium and potassium, we will observe that sodium belongs to period 3 and potassium belongs to period 4
This implies that Sodium has 3 shells and potassium has 4 shells. Hence, the ionic radius of potassium is larger than the ionic radius of sodium.
PART B
The electronegativity difference explains which bond is more ionic. Due to the structure of potassium and sodium, potassium chloride (KCl) has a higher electronegativity and the size of potassium (K) is greater than sodium (Na) atom. Hence, (K is more electrostatic than Na).
Therefore, KCl has stronger bonding than NaCl
The nucleus contains protons and neutrons while the electron revolves around the shell.
Recall that, both sodium and potassium are alkali metals and have the same chemical properties
The electronegativity of Potassium = 0.82
The electronegativity of sodium = 0.93
The electronegativity of chlorine = 3.16
The electronegativity difference of Nacl = 3.16 - 0.93
The electronegativity difference of NaCl = 2.23
The electronegativity difference of KCl = 3.16 - 0.82
The electronegativity difference of KCl = 2.34
Hence, the electronegativity difference of KCl is higher than the NaCl
NaCl has stronger bonding than KCl due to greater lattice energy
The decrease in the size of atom results in an increase in lattice energy. The size of a sodium atom is small compared to the size of a potassium atom because sodium has three shells and potassium has 4 shells. A decrease in the size of an atom leads to an increase in lattice energy. Hence, stronger bonding is a function of lattice energy.
Therefore, NaCl has stronger bonding than KCl
2H2O = 2H2 +O2
If 30mL of hydrogen are produced in above reaction, how many mL of oxygen are produced? Please show work.
Answers
Answer:
15 mL of O₂.
Explanation:
The balanced equation for the reaction is given below:
2H₂O —> 2H₂ + O₂
From the balanced equation above,
2 mL of H₂O reacted to produce 2 mL of H₂ and 1 mL of O₂.
Next, we shall determine the volume of H₂O needed to produce 30 mL of H₂. This can be obtained as follow:
From the balanced equation above,
2 mL of H₂O reacted to produce 2 mL of H₂.
Therefore, 30 mL of H₂O will also react to produce 30 mL of H₂.
Thus, 30 mL of H₂O is needed for the reaction.
Finally, we shall determine the volume of O₂ produced from the reaction. This can be obtained as follow:
From the balanced equation above,
2 mL of H₂O reacted to produce 1 mL of O₂.
Therefore, 30 mL of H₂O will react to produce = (30 × 1)/2 = 30/2 = 15 mL of O₂.
Thus, 15 mL of O₂ were produced from the reaction.
what happens when muscle cells are triggered?
Answers
Answer:
When myocytes (aka muscle cells) are triggered at a certain point for awhile it can cause strain and pain throughout the muscle.
. periodic table
* sub-shell
Diagrammatic questions
What factors do the valencies of elements depend upon?
A part of a periodic table is given below. Study it and answer the follow
ii. Sketch the atomic structure of that element which is placed in period 3
vi. transition element
IIA
IVA
IIIA
IA
VA
Be B. С N
Li
O
Periods 2
Mg
Si
Al
3. Na
Р S СІ
i Write the names of two very reactive non-metals.
questions.
VIA VIIA
O
Ne
F
Ar
has valency 4.
ii. Name the most reactive metal with a suitable reason.
study the given electronic configuration of the elements and answer the follo
questions.
A. 2,8,1 B.2,8 C. 2,8,7 D. 1
Answers
Answer:
Explanafgr7tfgt4ttion:
combustion always result in to formation of water. what other type of reactions may result into formation of water? examples of these reactions
Answers
As combustion always result into the formation of water, the other type of reactions that may result into formation of water are Acid-Base Neutralization Reactions and Hydrogen and Oxygen Reaction.
Acid-Base Neutralization Reactions:
A neutralisation reaction is a chemical process in which an acid and a base combine to produce salt and water as the end products.
H⁺ ions and OH⁻ ions combine to generate water during a neutralisation reaction. Acid-base neutralisation is the most common type of neutralisation reaction.
Example: Formation of Sodium Chloride (Common Salt):
HCl + NaOH → NaCl + H₂O
Hydrogen and Oxygen Reaction:
Water vapour is created when hydrogen gas (H₂) and oxygen gas (O₂) are combined directly. This reaction produces a lot of heat and releases a lot of energy.
Example: 2 H₂ + O₂ → 2 H₂O
Learn more about reactions:
https://brainly.com/question/25769000
What sample at STP has the same number of molecules as 5 L of NO2
Answers
Answer:
5l NO
2
at STP
No. of molecules=
22.4
5
mol=
22.4
5
×N
A
molecules
A) 5ℊ of H
2
(g)
No. of moles=
2
5
mol=
2
5
×N
A
molecules
B) 5l of CH
4
(g)
No. of moles of CH
4
=
22.4
5
mol=
22.4
5
N
A
molecules
C) 5 mol of O
2
=5N
A
O
2
molecules
D) 5×10
23
molecules of CO
2
(g)
Molecules of 5l NO
2
(g) at STP=5l of CH
4
(g) molecules at STP
Therefore, option B is correct.
Was this answer helpful?
where are chemicals found in the home?
a. only in the bathroom
b. only in locked cabinets
c. in every room
d. only in the kitchen
Answers
Answer:
c
Explanation:
chemicals can be found in every part of our lives
7) How many molecules of CO2 are in 2.5 L at STP?
Answers
By using the ideal gas law and Avogadro's number, we find that there are approximately 6.72 × 10^22 molecules of CO2 in 2.5 L at STP.
To determine the number of molecules of CO2 in 2.5 L at STP (Standard Temperature and Pressure), we can use the ideal gas law and Avogadro's number.
Avogadro's number (N_A) is a fundamental constant representing the number of particles (atoms, molecules, ions) in one mole of substance. Its value is approximately 6.022 × 10^23 particles/mol.
STP conditions are defined as a temperature of 273.15 K (0 °C) and a pressure of 1 atmosphere (1 atm).
First, we need to convert the volume from liters to moles of CO2. To do this, we use the ideal gas law equation:
PV = nRT,
where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
Since we have STP conditions, we can substitute the values:
(1 atm) × (2.5 L) = n × (0.0821 L·atm/(mol·K)) × (273.15 K).
Simplifying the equation:
2.5 = n × 22.4149.
Solving for n (the number of moles):
n = 2.5 / 22.4149 ≈ 0.1116 moles.
Next, we can calculate the number of molecules using Avogadro's number:
Number of molecules = n × N_A.
Number of molecules = 0.1116 moles × (6.022 × 10^23 particles/mol).
Number of molecules ≈ 6.72 × 10^22 molecules.
Therefore, there are approximately 6.72 × 10^22 molecules of CO2 in 2.5 L at STP.
For more such questions on ideal gas law visit:
https://brainly.com/question/27870704
#SPJ8
Solutions which can conduct electricity must contain:
atoms
minerals
molecules
ions
Answers
Answer
Ions
Explanation
Which are produced when HCl reacts with Ca(OH)2?
Cl2, H3, and CaO
CaCl and H3O
CaO, Cl2, and H2O
CaCl2 and H2O
Answers
Answer:
b did the test
Explanation:
did the test
A balanced chemical equation obeys the law of conservation of mass. The reaction of HCl with Ca(OH)₂ is a weak base - strong acid reaction. The products formed are CaCl₂ and H₂O. The correct option is D.
What is a balanced chemical equation?A chemical equation in which the number of reactants and products on both sides of the equation are equal is defined as the balanced chemical equation. The coefficients are the numbers which are added to balance the chemical equation.
The balanced equation for the reaction of HCl with Ca(OH)₂ is given as:
Ca (OH)₂ (s) + 2HCl (aq) → CaCl₂ (aq) + 2H₂O (l)
Here one mole of Ca (OH)₂ reacts with two moles of HCl and gives one mole of CaCl₂ and two moles of H₂O respectively. Here Ca (OH)₂ is a white precipitate and HCl is a aqueous solution.
Thus the correct option is D.
To know more about balanced chemical equation, visit;
https://brainly.com/question/15052184
#SPJ7
Space shuttles are made out of three main parts: rocket boosters, a fuel tank, and a(n) ___________.
Answers
Answer:
Orbiter
Explanation:
Space shuttles are made out of three main parts: rocket boosters, a fuel tank, and orbiter (the part that resembles an airplane
Calculate the pOH for a solution that has a hydroxide ion, (OH-) concentration of 5.02 X 10-4 M.
Calculate the pH.
Note: the answers should have three significant figures
Answers
Answer:
Explanation:
Calculate the pOH for a solution that has a hydroxide ion, (OH-) concentration of 5.02 X 10-4 M.
Calculate the pH.
Note: the answers should have three significant figures
Liquid water - heat =
Pls help now!!!
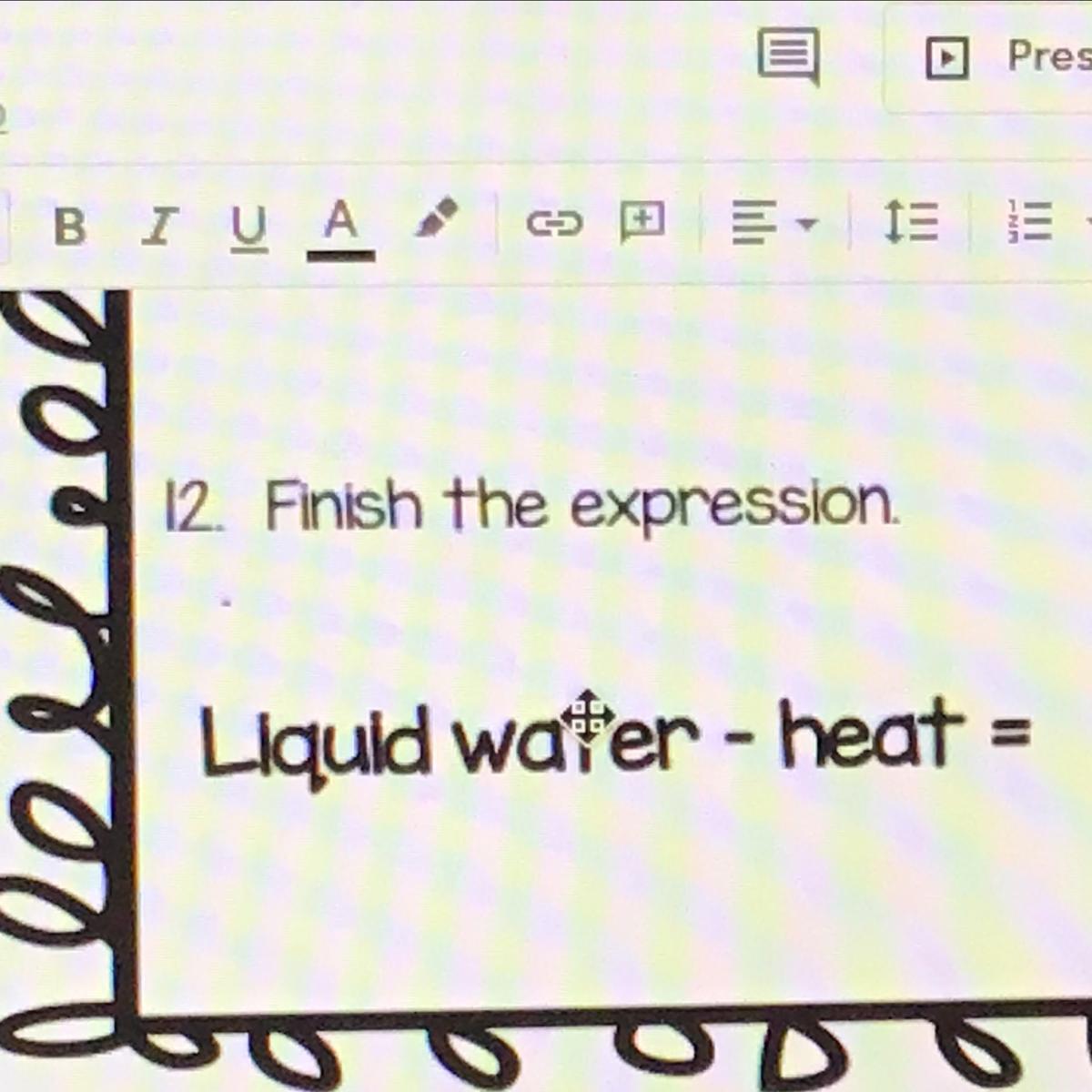
Answers
Answer:
cold or ice?
Explanation:
have a good day.
HELPPPPP. I NEED HELP. I NEED A CER ON THISSSS. I WILL PAY $5 or $10 I need help. Plzzzzz

Answers
Answer:
potassium and calcium
In which of the following reactions will Kc = Kp? a. 4 NH3(g) + 3 O2(g) ⇌ 2 N2(g) + 6 H2O(g) b. SO3(g) + NO(g) ⇌ SO2(g) + NO2(g) c. 2 N2(g) + O2(g) ⇌ 2 N2O(g) d. 2 SO2(g) + O2(g) ⇌ 2 SO3(g)
Answers
Answer:
The correct option is b) SO₃(g) + NO(g) ⇌ SO₂(g) + NO₂(g)
Explanation:
The relation between Kc and Kp is given by the following equation:
\(Kp = Kc (RT)^{dn}\)
where R is the gas constant (0,082 L.atm/K.mol), T is the temperature (in K) and dn is the change in moles.
The change in moles (dn) is calculated as:
dn = moles of products - moles reactants
If dn=0, RT= 1 ⇒ Kc=Kp
We calculate dn for each reaction from the estequiometrial coefficients of products and reactants as follows:
a) 4 NH₃(g) + 3 O₂(g) ⇌ 2 N₂(g) + 6 H₂O(g)
dn= (2+6) - (4+3) = 1 ⇒ Kc ≠ Kp
b) SO₃(g) + NO(g) ⇌ SO₂(g) + NO₂(g)
dn = (1+1) - (1+1)= 0 ⇒ Kc = Kp
c) 2 N₂(g) + O₂(g) ⇌ 2 N₂O(g)
dn= 2 - (2+1) = -1 ⇒ Kc ≠ Kp
d) 2 SO₂(g) + O₂(g) ⇌ 2 SO₃(g)
dn = 2 - (2+1) = -1 ⇒ Kc ≠ Kp
The reaction in which Kc=Kp is b), because reactants and products have the same number of moles.
An earthquake creates a type of wave that shakes the ground. If a large earthquake occurs in Greece, how can the waves be felt across the sea in Italy?
A.
Waves can be transformed into strong winds that travel across the sea.
B.
Waves do not travel through water, but they can crash the sea into land far away.
C.
Waves can cause a series of earthquakes around the world.
D.
Waves can travel through different media including solid land and water.
Answers
1.1
A sample of oxygen occupies 47.2 L under a pressure of 1240 torr at 25 °C. What
volume would it occupy at 25 °C if the pressure were decreased to 730 torr?
Answers
Answer:
80.2L
Explanation:
Given parameters:
Initial volume of oxygen = 47.2L
Initial pressure of oxygen = 1240torr
Final pressure = 730torr
Unknown:
Final volume = ?
Solution:
To solve this problem, we apply the Boyle's law proposed by Robert Boyle. It states that "the volume of a fixed mass of a gas varies inversely as the pressure changes if the temperature is constant".
Mathematically;
P₁V₁ = P₂V₂
P and V are pressure and volume
1 and 2 are initial and final states
1240 x 47.2 = 730 x V₂
V₂ = 80.2L
Which of the following statement is not true about derl ton's atomic theory
Answers
A botfly lays an egg in the skin of an animal. When the larvae hatches from the egg, it burrows deep into the skin . The animal may become sick from having the larvae under its skin. What type of relationship is being describe, and what roles do each organism play?
Answers
Answer:
A botfly that lays an egg in the skin of an animal when the larvae hatches from the egg, it burrows deep into the skin. The animal that may become sick from having the larvae under its skin is called Mutualism.
Explanation:
The animal is the host while the botfly is the parasite. A mutualism in which one mutualistic partner removes parasites, as well as dead or diseased skin from another, in return receiving a steady supply of food is called Pollination. Nearly all pollination services involve a mutualism that has evolved over millions of years. Another example is reproduction.
a 2.72 g sample of a compound consisting of carbon, hydrogen, oxygen, nitrogen, and sulfur was combusted in excess oxygen. this produced 1.53 g co2 and 0.940 g h2o . a second sample of this compound with a mass of 6.37 g produced 6.53 g so3 . a third sample of this compound with a mass of 8.43 g produced 3.40 g hno3 . determine the empirical formula of the compound. enter the correct subscripts on the given chemical formula.
Answers
The empirical formula of the compound is C10H15N5O15S.
To determine the empirical formula of the compound, we need to calculate the mass of each element in the compound and then find the ratios of the elements.
Let's start with the combustion analysis:
Mass of CO2 produced = 1.53 g
Mass of H2O produced = 0.940 g
Using the molar masses of CO2 (44.01 g/mol) and H2O (18.02 g/mol), we can calculate the moles of carbon and hydrogen in the compound:
Moles of carbon = 1.53 g / 44.01 g/mol = 0.0348 mol
Moles of hydrogen = 0.940 g / 18.02 g/mol = 0.0521 mol
Next, let's consider the reaction with SO3:
Mass of compound = 6.37 g
Mass of SO3 produced = 6.53 g
Using the molar mass of SO3 (80.06 g/mol), we can calculate the moles of sulfur in the compound:
Moles of sulfur = 6.53 g / 80.06 g/mol = 0.0816 mol
Finally, let's consider the reaction with HNO3:
Mass of compound = 8.43 g
Mass of HNO3 produced = 3.40 g
Using the molar mass of HNO3 (63.01 g/mol), we can calculate the moles of nitrogen in the compound:
Moles of nitrogen = 3.40 g / 63.01 g/mol = 0.054 mol
We also know that the total mass of the compound is 2.72 g + 6.37 g + 8.43 g = 17.52 g.
Now we can find the empirical formula of the compound by dividing the moles of each element by the smallest number of moles. The smallest number of moles is 0.0348 mol, which corresponds to the carbon atoms in the compound.
Empirical formula = C1H1.5N0.5O1.5S0.1
To get whole number subscripts, we can multiply all the subscripts by 10:
Empirical formula = C10H15N5O15S
To know more about empirical formula here
https://brainly.com/question/28116165
#SPJ4
How many grams of AuCl3 contain 5.0 x 1023 molecules?
Answers
Answer:
approximately 251.55 grams of AuCl3 would contain 5.0 x 10^23 molecules.
after the equilibrium represented above is established, some pure o2(g) is injected into the reaction vessel at constant temperature. after equilibrium is reestablished, which of the following has a lower value compared to its value at the original equilibrium?
Answers
The amount of SO₂(g) in the reaction vessel has a lower value compared to its value at the original equilibrium. Option d is correct choice.
Injecting pure O₂(g) into the reaction vessel at a constant temperature will cause the system to shift towards the right side of the equation in order to consume the added O₂. This means that the amount of SO₂(g) in the reaction vessel will decrease, while the amount of SO₃(g) and O₂(g) will increase.
Since the reaction is exothermic, adding O₂(g) will not change the equilibrium constant (Keq) of the reaction, so (A) is not affected. However, the amount of O₂(g) in the reaction vessel will increase, so (C) has a higher value compared to its value at the original equilibrium.
To know more about the equilibrium, here
brainly.com/question/23975996
#SPJ4
--The complete question is, 2SO(g) <-> 2SO₂(g) + O₂(g)
After the eq. represented above is established, some pure O₂(g) is injected into the reaction vessel at a constant temperature. After equilibrium is reestablished, which of the following has a lower value compared to its value at the original equilibrium?
A. Keq for the reaction
B. The amount of SO₃(g) in the reaction vessel
C. The amount of O₂(g) in the reaction vessel.
D. The amount of SO₂(g) in the reaction vessel.--
When do chemical reactions happen? *

Answers
Observe: Turn on Show molecular view, and notice the water molecules. Set the Water volume to 100 mL and the Powder mass to 20 g, and then click Play. Click Pause () after adding the powder. You should now see show some sodium acetate in the water.
What color represents the bonds between the particles of NaC2H3O2?
Click Play. Watch the animation a few times. What happens to the NaC2H3O2 bonds?
What happens to the bonds between water molecules?
Answers
Answer: how old are you
Explanation:
is hydrogen peroxide a product
Answers
_________ is when humans choose the traits of an organism.
Answers
Your answer should be -selective breeding
\(hope that helps\)
One mole of Oxygen molecule contain how many atoms of oxygen
Answers
Answer:
6.02 X 1023 atoms of oxygen.
Explanation:
One mole of atoms of oxygen has a mass of 16 g, as 16 is the atomic weight of oxygen, and contains 6.02 X 1023 atoms of oxygen.
the number of oxygen atoms an oxygen molecule has is two oxygen atoms
The structure of a disaccharide is shown below; Which statement applies? Cnioh Oloh cilolia Gletute
Only the Glucose ring is in equillbrium with a open chain form
Both the Glucose and Fructose rings are in equllibrlum wlth an open chaln form:
Only the Fructose ring Is In equlllbrium wlin an open chain form
Neither ring Is In equlllbrium wuth an open chain form,

Answers
While either glucose and fructose circles are in this state, only the fructose rings are still in equilibrium with only an extended chain form.
What does a glucose do?The major source of energy for the body's cells is glucose, which is present in the blood as sugar. The body can make glucose from those other molecules or it can get it from the food we eat. Glucose is transported to the cells by the circulation. One hormone that controls blood glucose levels is insulin.
What foods are high in glucose?Some foods that are naturally high in pure glucose include sweet maize, syrup, agave syrup, dried apricots, fruits, and fruit juices. when included in a well-balanced food and consumed in moderation.
To know more about glucose visit:
brainly.com/question/2396657
#SPJ4
98.96g/mol of CH2O what will be the chemical formula
Answers
Let's break down the molar mass of CH2O:
- Carbon (C) has a molar mass of approximately 12.01 g/mol.
- Hydrogen (H) has a molar mass of approximately 1.01 g/mol.
- Oxygen (O) has a molar mass of approximately 16.00 g/mol.
Now, let's calculate the molar mass of CH2O:
(1 x molar mass of C) + (2 x molar mass of H) + (1 x molar mass of O)
= (1 x 12.01 g/mol) + (2 x 1.01 g/mol) + (1 x 16.00 g/mol)
= 12.01 g/mol + 2.02 g/mol + 16.00 g/mol
= 30.03 g/mol
The molar mass of CH2O is approximately 30.03 g/mol, which is different from the given molar mass of 98.96 g/mol.
It seems that there might be an error or misunderstanding in the given molar mass value. The correct chemical formula for a compound with a molar mass of 98.96 g/mol cannot be determined based on the information provided.