Answers
Answer:
2a. Calcium chloride
2b.hydrochloride acid
2c. water molecules
2d.hydrochloric acid
2e.carbon dioxide
Explanation:
CaCO3+2HCl=CaCl2+CO2+H2O
Related Questions
What are the characteristics of nonvascular plants? (select all that apply) A . They don’t rely on roots to receive water.
B .They have xylem vessels and phloem vessels
C . They are able to push water and nutrients to each part of the plant.
D . They are found in moist environments.
Answers
Answer:
D . They are found in moist environments.
Explanation:
Nonvascular plants do not have a xylem or phloem, roots, stems, or leaves. Because these plants lack water-conducting tissues, they fail to achieve the structural complexity and size of most vascular plants and have evolved in habitats which allow their survival and reproduction.
The plant body that is most obvious in non-vascular plants are the the gametophyte generation. The gametophte gemeration is haploid.
The non-vascular plants grow in moist environments. It is due to lack of vascular tissue that requires to maintain close contact with water to prevent desiccation. Nonvascular plants are plants that do not have any special internal pipelines or channels to carry water and nutrients. Instead, nonvascular plants absorb water and minerals directly through their leaflike scales. Nonvascular plants are usually found growing close to the ground in damp, moist places. Non-vascular plants thrive in damp conditions since they don't need to rely on roots to acquire enough water.
A chemist prepares a solution of silver(I) nitrate AgNO3 by measuring out 62.3μmol of silver(I) nitrate into a 50.mL volumetric flask and filling the flask to the mark with water.
Calculate the concentration in /molL of the chemist's silver(I) nitrate solution. Be sure your answer has the correct number of significant digit
*please write the answer without any files disturbance*
Answers
Answer:
0.0012 mol/L.
Explanation:
From the question given above, the following data were obtained:
Number of mole of AgNO₃ = 62.3 μmol
Volume = 50 mL
Molarity of AgNO₃ =?
Next, we shall convert 62.3 μmol to mole. This can be obtained as follow as follow:
1 μmol = 10¯⁶ mole
Therefore,
62.3 μmol = 62.3 × 10¯⁶
62.3 μmol = 62.3×10¯⁶ mole
Next, we shall convert 50 mL to L. This can be obtained as follow:
1000 mL = 1 L
Therefore,
50 mL = 50 mL × 1 L / 1000 mL
50 mL = 0.05 L
Finally, we shall determine the concentration of AgNO₃ in mol/L as follow:
Number of mole of AgNO₃ = 62.3×10¯⁶ mole
Volume = 0.05 L
Molarity of AgNO₃ =?
Molarity = mole /Volume
Molarity of AgNO₃ = 62.3×10¯⁶ / 0.05
Molarity of AgNO₃ = 0.0012 mol/L
Thus, the concentration of AgNO₃ is 0.0012 mol/L
Why are cats better than dogs?
Answers
Answer:
Cats are eazy to take care for and relatively affordable
Answer:
cats r better cuz there cats ovi
Explanation:
there small, easy to take care of, and the are good with kids depending on the breed.
2. A gas that occupies a volume of 6.75 L at 89.0 atm will occupy what volume at 68.55 mm Hg if the temperature remains constant?
Answers
First convert pressure to atm
1atm=760mm hg68.55 mm Hg= 0.09atmNow
V1=6.75LV2=?P1=89atmP2=0.09atmApply Boyle's law
\(\\ \rm\Rrightarrow P1V1=P2V2\)
\(\\ \rm\Rrightarrow 89(6.75)=0.09V2\)
\(\\ \rm\Rrightarrow 600.75=0.09V2\)
\(\\ \rm\Rrightarrow V2=\dfrac{600.75}{0.09}\)
\(\\ \rm\Rrightarrow V2=6675L\)
0.10 molL-1 NaCl solution contains 1.0 mole of NaCl
Answers
The volume of the 0.10 molL⁻¹ NaCl solution which contains 0.1 mole of sodium chloride, NaCl is 1 L
How do i determine the volume of the solution?Molarity of a solution is defined by the following formula:
Molarity = mole / volume
Cross multiply
Molarity × volume = Mole
Divide both sides by molarity
Volume = mole / molarity
With the above formula, we can obtain the volume of the solution. Details below:
Molarity of solution = 0.10 molL⁻¹Mole of NaCl = 0.1 moleVolume of solution =?Volume = mole / molarity
Volume of solution = 0.1 / 0.1
Volume of solution = 1 L
Thus, we can conclude that the volume of the solution is 1 L
Learn more about volume:
https://brainly.com/question/29144710
#SPJ1
Complete question:
0.10 molL⁻¹ NaCl solution contains 1.0 mole of NaCl. What is the volume of the solution?
Scientific theories are subject to change.
Answers
Answer:
true
Explanation:
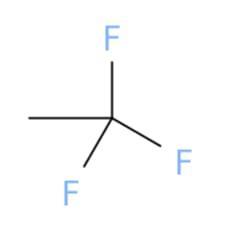
Select the correct structure that
corresponds to the name.
1,1,1-trifluoroethane

Answers
The correct chemical structure that corresponds to 1,1,1-trifluoroethane is (a).
What is 1,1,1-trifluoroethane?
A chemical structure is a spatial arrangement of atoms in a molecule. It determines the molecular geometry and when necessary the electronic chemistry as well .1,1,1-Trifluoroethane or simply known as trifluoroethane is Hydrofluorocarbon (HFC) compound that is colourless and highly inflammable gas with ether like odour. One method of preparation of 1,1,1-Trifluoroethane is by fluorination of 1-chloro-1,1-difluoroethane in the presence of hydrofluoric acid. The chemical formula for 1,1,1-Trifluoroethane is \(C__{2} } H_{3} F_{3}\). The high stability of it's chemical structure because of being heavier than air makes it a greenhouse gas with high infrared absorbent power. It can be used as a propellant or refrigerant and in cleaning of electrical equipments.
Learn more about 1,1,1-trifluoroethane here:
https://brainly.com/question/1390779
#SPJ1
How many carbon dioxide molecules should be added to the products side to obey the law of conservation of matter?
Your answer:
A.four
B.One
C.three
D.two

Answers
Answer:
four A
Explanation:
Answer: B) One
Explanation: One carbon dioxide molecule should be added to the products side to balance the equation and obey the law of conservation of matter.
Also, just took the test. One is correct.
PLEASE CHECK MY ANSWERS AND VERIFY THEM! THANK YOU SO MUCH !!!!!

Answers
PLEASE HELP, IS MY ANSWER CORRECT?
How does the ground temperature in sunlight with CO2 compare with the ground temperature in sunlight without CO2 (part A)? is my answer correct?
Based on the thermometer provided, it is clearly visible that when the simulation is without CO2, the temperature goes higher, however, not as quickly as when CO2 IS present.
Answers
Based on the thermometer provided, the ground temperature in sunlight with CO2 rises more rapidly and reaches a higher temperature compared to the ground temperature in sunlight without CO2.
Calculate the percent composition of H3PO4
Answers
The percent composition of hydrogen, phosphorous and oxygen in phosphoric acid is 3.06 %,31.60%,65.31 % respectively.
What is percent composition?Percent composition is defined as a convenient way to record concentration of solution.It is a expression which relates solute to solvent as,mass of solute/mass of solution ×100.There are two types of percentage composition percent weight by volume and percent volume by volume .
Percent composition of hydrogen=3/97.99×100=3.06%.
Percent composition of phosphorous=30.97/97.99×100=31.60%
Percent composition of oxygen=64/97.99×100=65.31%
Learn more about percent composition,here:
https://brainly.com/question/17505281
#SPJ1
Definition of specific heat
Answers
Answer and Explanation:
Heat required to raise the temperature of the unit mass of a given substance by a given amount (usually one degree).
Answer:
the heat required to raise the temperature of the unit mass of a given substance by a given amount (usually one degree)
Hope it helps!!
An object with a mass of 0.255 kg and density of 2.89 g/cm^3 measures 34 mm in length and 46 mm in width. What is the height of the object?
1) 5.6 cm
2) 5.6•10^-2 cm
3) 7.2 •10^-4 cm
Answers
5.64 I think, I'm sorry if I'm wrong
Which of the following is not a product of the reaction of zinc metal with nitric acid?
A. H2O
B. Zn2+
C. H2
D. NH4+
E. All of the above species are products.
Answers
Answer:
A. H2O
Explanation:
The balanced ionic equation for the reaction is:
\(Zn^{2+} + 2H^{+} NO_{3} ^{-1}\) → \(Zn^{2+} (NO_{3} )^{-1} _{2} + H^{2+} _{2}\)
This implies that the products of the reaction are zinc nitrate and hydrogen gas only.
Therefore, the outlier from the options is water (H2O)
Let me know if you have any other questions.
is osmosis water diffuses towards the solution, that is, toward the solution with the solute concentration
Answers
Answer:
Yes, osmosis is the process by which water moves across a semi-permeable membrane from a region of lower solute concentration to a region of higher solute concentration. This is driven by the difference in solute concentration on either side of the membrane.
What is the best description of blood.
(a)sol (b) solution (c)foam (d) liquid aerosol
Answers
Answer:
Explanation:
liquid
How many water molecules are found within the crystalline structure of one hydrate molecule?
What is the molecular formula of the hydrate?
Attached my worksheets to it and the questions


Answers
a. The Mass of water driven off = 0.15 g
b. Moles of anhydrate = 0.00257 moles
c. Moles of water driven off is 0.00833 moles
d. There are 3 moles of water within the crystalline structure of one molecule of the hydrated salt.
e. The molecular formula of the hydrated salt will be X.3H₂O
What is the mass of water driven off from the hydrated salt?a. The mass of water driven off from the hydrated salt is:
Mass of water driven off = 0.5 g - 0.35 g
Mass of water driven off = 0.15 g
b. Molecular mass of salt = 136 g/mol
moles of anhydrate = 0.35/136
Moles of anhydrate = 0.00257 moles
c. Moles of water driven off = mass/molar mass
molar mass of water = 18 g/mol
Moles of water driven off = 0.15/18
Moles of water driven off = 0.00833 moles
d. Moles of water within the crystalline structure of one molecule of the hydrated salt is determined by converting to whole number mole ratio by dividing with the smallest ratio,
Salt to water ratio = 0.00257 /0.00257 : 0.00833/0.00257
Salt to water ratio = 1 : 3
Therefore, there are 3 moles of water within the crystalline structure of one molecule of the hydrated salt.
e. Assuming the anhydrous salt is X, the molecular formula of the hydrated salt will be X.3H₂O
Learn more about hydrated salts at: https://brainly.com/question/14447094
#SPJ1
Use the periodic table or graphic in lesson. Choose the correct electron configuration of carbon. 1s 22s 22p 4 1s 22s 22p 2 1s 22s 22p 1 1s 22s 12p 2
Answers
The Correct electron configuration of carbon as 1s² 2s² 2p². Option B.
To understand why this is the correct electron configuration, let's break it down step by step:
The atomic number of carbon is 6, which means it has six electrons. Electrons are distributed in energy levels or shells around the nucleus.
The first shell, known as the 1s orbital, can hold a maximum of 2 electrons. Therefore, the first part of the electron configuration is 1s², indicating that two electrons occupy the 1s orbital.
The second shell has two subshells: the 2s orbital and the 2p orbital. The 2s orbital can hold a maximum of 2 electrons, while the 2p orbital can hold a maximum of 6 electrons. In the case of carbon, after the 1s orbital, two more electrons occupy the 2s orbital. So far, we have 1s² 2s².
The remaining two electrons in carbon are placed in the 2p orbital. The 2p orbital consists of three separate p orbitals: 2px, 2py, and 2pz. Each p orbital can hold a maximum of 2 electrons. Therefore, the last part of the electron configuration for carbon is 2p², indicating that two electrons occupy the 2px and 2py orbitals. Option B is correct.
For more such question on electron. visit :
https://brainly.com/question/28420589
#SPJ8
At 25°C, an aqueous solution containing 35.0 wt% H2SO4 has a specific gravity of 1.2563. A quantity of the 35% solution is needed that contains 195.5 kg of H2SO4.
A) Calculate the required volume (L) of the solution using the given specific gravity.
B) Estimate the percentage error that would have resulted if pure-component specific gravities of H2SO4 (SG = 1:8255) and water had been used for the calculation instead of the given specific gravity of the mixture.
Answers
Answer:
a) volume₁ = 444.6 L
b) Volume₂ = 306 L and percentage Error = 31.2%
Explanation:
Given that;
the solution contains 35.0 wt% H₂SO₄
A quantity of the 35% solution is needed that contains 195.5 kg of H₂SO₄
Lets say mass of solution containing 195.5 kg H₂SO₄ is 'A' kg
Now since the question saysm it is a 35% wt solution,
so
(35/ 100) × Akg = 195.5kg
0.35A = 195.5
A = 558.6kg
So A = 558.6 kg
therefore mass of the solution is 558.6kg
a)
also Specific gravity is 1.2563
since density of water = 1kg/ L
density of solution = SG of H₂SO₄ × density of water
therefore density of solution = 1.2563 ×1kg/ L = 1.2563 kg/ L
Now to calculate the required volume (L) of the solution
we say;
Volume of solution = mass / density
Volume = 558.6kg / 1.2563kg/L
Volume₁ = 444.6 L
b)
Now If pure-component specific gravity is to be used,
Specific Gravity = 1.8255
which means Density will be = 1.8255 kg/ L
Therefore will be
Volume = 558.6kg / 1.8255kg/L
Volume₂ = 306 L
To calculate the error
we say volume₁ - volume₂
Error = 444.6L - 306L = 138.6
So
Percent error = ( 138.6L / 444.6L) × 100
percentage Error = 31.2%
you are working on a project where you need to find the volume of a box. you take yhe length, height, and width measurements and then multiply your values together to find the volume. you report the bolume of the box as .310m^3. if two of your measuremnets were .7120m and .52485m, what was the other measurement
Answers
Explanation:
First find the value sheet and send the original file for me
Step by step
We know L x W x H = .310 m^3
We have two of the box lengths to fill in
.7120 x .52485 x ( H unknown) = .310 m^3
Multiply
.3736932 H = .310 m^3
Divide both sides by 37369.32 to solve for H
.3736932/ .3736932 H = .310/ .3736932
H = .82955751 meters is your answer
Check your work
.7120 x .52485 x .82955751 = .310 m ^3
Problem solved!
How many grams of H2SO4 are needed to prepare 5.0 L of a 2 M H2SO4 solution? You must show your work in order to receive credit.
Answers
To prepare 5L of a 2.0M solution you require: 98g/mol * 5mol * 2mol /L = 980g H2SO4
ACTIVITY: SOLUTION CONCENTRATION VS. CONDUCTIVITY
Here is your goal for this lesson:
Graph experimental data and interpret results for peer review
A chemistry student carried out an experiment with a conducting apparatus (ammeter) similar to the one below. This ammeter measures in milliamperes (mA). The following data was taken.
Solution Reading
0.1 M H2SO4 150 mA
0.1 M Ba(OH)2 150 mA
To 30 mL of the Ba(OH)2 solution, 10 mL portions of H2SO4 were added until a total of 50 mL of H2SO4 were used. The following results were recorded.
DATA TABLE
Total H2SO4 Added Meter Reading Observations
0 mL 150 mA Ba(OH)2 and H2SO4 clear, colorless
10 mL 65 mA milky white precipitate forms
20 mL 31 mA more precipitate forms
30 mL 0 mA precipitate heavy and settles
40 mL 29 mA no added precipitate seen to form
50 mL 62 mA no change seen
Questions
1. Did you plot the data?
yes
no
2. Did you label your graph axes?
no
yes
3. Did you give your graph a title?
no
yes
4. Does the Ba(OH)2 solution contain ions?
yes
no
5. Does the H2SO4 solution contain ions?
yes
no
6. Explain the data.
Is there any evidence that a reaction has occurred?
7. Does the conductivity increase or decrease?
8. Does the number of ions in solution increase, decrease, or remain constant?
9. What is the indicator of the number of ions in solution?
10. How does this evidence indicate that the reaction has occurred between ions?
11. The Ba(OH)2 dissociates as Ba+2 + 2 OH-. H2SO4 dissociates as 2 H+ + SO4-2.
Write a balanced equation for this reaction.
12. When the conductivity is at a minimum, what must be true about the amount of Ba(OH)2?
13. Why does it not conduct at this low point?
14. Why does it conduct more before and after this minimum point?

Answers
Answer:
the amount of Ba(OH)2 compared to H2SO4?
The Ba(OH)2 dissociates as Ba+2 + 2 OH-. H2SO4 dissociates as 2 H+ + SO4-2.
Why does it not conduct at this low point?
Why does it conduct more before and after this minimum point
Can someone please help with this I am not that great at chemistry. Thankyou fo any help that I ge
How much heat would be required to convert 5.31 mol of a pure substance from a liquid at 40.0°C to a gas at 113.0°C?
Answers
Answer:
Q = 7.7 KJ
Explanation:
The complete question carries the following data:
Specific Heat Capacity of Liquid State = C(l) = 1.45 J/mol.°C
Specific Heat Capacity of Gaseous State = C(g) = 0.65 J/mol.°C
Boiling Temperature = Tb = 88.5°C
Heat of Vaporization = ΔH(vap) = 1.23 KJ/mol
Now, first we calculate the heat required (Q₁) to raise he temperature to boiling point:
Q₁ = n*C(l)*ΔT₁
where,
n = no. of moles = 5.31 mol
ΔT₁ = Temperature difference from 40°C to Tb = 88.5°C - 40°C = 48.5°C
Therefore,
Q₁ = (5.31 mol)(1.45 J/mol.°C)(48.5°C)
Q₁ = 373.4 J = 0.37 KJ
Now, we calculate the heat required (Q₂) to change its phase:
Q₂ = nΔH(vap)
Q₂ = (5.31 mol)(1.23 KJ/mol)
Q₂ = 6.53 KJ
Now, we calculate the heat required (Q₃) to raise he temperature to boiling point:
Q₃ = n*C(g)*ΔT₃
where,
n = no. of moles = 5.31 mol
ΔT₃ = Temperature difference from Tb to 113°C = 113°C - 88.5°C = 24.5°C
Therefore,
Q₃ = (5.31 mol)(0.65 J/mol.°C)(24.5°C)
Q₃ = 84.5 J = 0.08 KJ
So, the total heat required to convert 5.31 mol of this pure substance from a liquid at 40°C to a gas at 113°C is:
Q = Q₁ + Q₂ + Q₃
Q = 0.37 KJ + 6.53 KJ + 0.08 KJ
Q = 7.7 KJ
The total heat required to convert the pure substance from liquid to gas at the given temperature is 814 J.
The given parameters;
molar heat capacity of liquid, C(l) = 1.45 J/mol.⁰Cmolar heat capacity of gas, C(g) = 0.65 J/mol.⁰CThe total heat required to convert the pure substance from liquid to gas at the given temperature is calculated as follows;
\(Q = nc_l \Delta \theta + nc_g \Delta \theta \\\\ \Delta \theta = (113 - 40) = 73^0 C \\\\Q = (5.31 \ mol \times 1.45 \frac{J}{mol .\ ^0C} \times 73\ ^0C) + (5.31 \ mol \times 0.65 \frac{J}{mol .\ ^0C} \times 73 \ ^0C)\\\\Q = 814 \ J\)
Thus, the total heat required to convert the pure substance from liquid to gas at the given temperature is 814 J.
"Your question is not complete, it seems to be missing the following information;"
How much heat would be required to convert 5.31 mol of a pure substance from a liquid at 40.0°C to a gas at 113.0°C? (molar heat capacity of liquid, C(l) is 1.45 J/mol.⁰C and molar heat capacity of gas, C(g) is 0.65 J/mol.⁰C).
Learn more here:https://brainly.com/question/8428922
Why do human reproductive cells (i.e. egg & sperm cells) only end up with 23 chromosomes by the end of meiosis cell division?
A. Meiosis only does part of the job of reproductive cell division, and another type of cell division has to finish it.
B. Each reproductive cell gets only half the genetic code of a complete human so that when they combine during sexual reproduction, it results in a
human with a full genetic code.
Answers
Only half of a human's genetic code is received by each reproductive cell, ensuring that when they join during sexual reproduction, a human with a full genetic code is produced.
Why only have 23 chromosomes in sperm and egg cells, the gametes?Human sperm and eggs must only have 23 chromosomes for fertilization to result in the formation of a single, fully developed diploid cell that will then divide through mitosis to become a whole organism.
Why do gametes, or sperm and egg cells created during meiosis, have half as many chromosomes as they should?This decrease in chromosome number is essential for meiosis because it produces cells that will develop into gametes (or reproductive cells); without it, fertilization cannot result in the union of two gametes.
To know more about chromosomes visit:-
https://brainly.com/question/1596925
#SPJ1
Which is NOT a disadvantage of using electromagnetism?
A.) cause cancer
B.) damage medical equipment
C.) accurate viewing of soft tissue in the body
D.) heat up quickly
Answers
Answer:
Electromagnetism can cause cancer
So option A is incorrect
Electromagnetism can damage a medical instruments
It is also a disadvantage and this option is not correct
Now come to C it says that electromagnetic is used to veiw the soft tissue it is not a disadvantage but a advantage so C IS THE CORRECT ANSWER
Explanation:
PLEASE MARK ME BRAINLIEST IF MY ANSWER IS CORRECT PLEASE
Calculate the number of moles of gas produced from the reaction of 2.00g of potassium with an excess amount of water.
Answers
The number of moles of gas produced from the reaction of 2.00g of potassium with an excess amount of water is 0.025 moles.
The reaction of potassium with an excess amount of water is:
2K + 2H\(_2\)O \(\rightarrow\) 2KOH + H\(_2\)
To calculate the moles of hydrogen gas first we need to calculate moles of potassium in 2.00g
No. of moles = (mass) / (molecular mass)
The mass given is 2.00 g and the Molecular mass is 39.09 units
∴ No. of moles = (2) / (39.09) = 0.05
From the above reaction, we get that 2 moles of potassium give 1 mole of hydrogen gas. Thus, 0.05 moles of potassium gives 0.025 moles of hydrogen gas.
Therefore, the no. of moles of hydrogen gas produced is 0.025 moles.
To learn more about potassium,
brainly.com/question/13321031
Which of the following statements correctly describe chemical kinetics? Select all that apply.Chemical kinetics describes chemical reactions using collision theory.Chemical kinetics is the study of reaction rates.Chemical kinetics measures how fast reactants are converted into products.
Answers
Understanding how quickly chemical reactions occur is the focus of the physical chemistry subfield known as chemical kinetics. In contrast to thermodynamics, which focuses on the direction in which a process takes place but says nothing about its rate, it is to be contrasted with this.
A chemical reaction is a procedure that causes one group of chemical components to change chemically into another. Chemical reactions, which can frequently be described by a chemical equation, traditionally include changes that only affect the locations of electrons in the formation and dissolution of chemical bonds between atoms, with no change to the nuclei (no change to the elements present). The study of chemical processes involving unstable and radioactive elements, where both electronic and nuclear changes may take place, is known as nuclear chemistry. Reactants or reagents are the substance(s) or substances that are initially utilized in a chemical reaction. Chemical reactions typically involve a chemical change and produce one or more products, which typically differ from the reactants in some ways. The details of the particular course of action are part of the reaction mechanism. Reactions frequently consist of a series of discrete steps, or elementary reactions. Chemical equations, which represent the initial substances, final products, and occasionally intermediate products as well as the reaction circumstances symbolically, are used to describe chemical reactions.
Learn more about chemical reactions here
https://brainly.com/question/19151122
#SPJ4
Select the conjugate acid-base pair(s). a) HI, I b) HCHO2, SO4^2- c) CO3^2-, HCI d) PO4^3-, HPO4^2-
Answers
Answer:
PO4^3-, HPO4^2-
Explanation:
If an acid gives out a proton, the acid then changes to its corresponding base. Similarly, if a base takes in a proton, it changes to its corresponding acid. If a pair of acid and base differ only by the presence or absence of a proton, then they are referred to as a conjugate acid-base pair.
Let us look at this;
HPO4^2-(aq) ----> H^+(aq) + PO4^3-(aq)
HPO4^2- and PO4^3- differ only in the presence or absence of a proton (H^+) hence they constitute a conjugate acid-base pair.
explain what is ment by solvent front
Answers
Answer:
In paper chromatography, the wet moving edge of the solvent that progresses along the surface where the separation of the mixture is occurring.
Explanation:
I hope this helps and pls mark me brainliest :)
heeeelp
Plants do not have bones that provide them with structure and support, they have
_____.
tubes made of cellulose
cytoplasm
an exoskeleton
muscles
Which of the following is a tendon and not a ligament?
The anterior cruciate ligament (ACL) which is located toward the front of the knee and controls forward movement and rotation of the surrounding bones.
The ulnar collateral ligaments which runs along the inner side of the elbow connecting bones
The anterior talofibular ligament which connects the talus (a bone in the foot) to the fibula (the outer bone in the leg).
The Achilles which connects calf muscles to your heel bone
Which type of organisms have an exoskeleton?
mammals
crustacean
worms
plants
All organisms have either an endoskeleton or an exoskeleton.
True
False
Answers
Plants do not have bones that provide them with structure and support, they have tubes made of cellulose; option A.
The Achilles is a tendon and not a ligament; option D
The type of organism that has an exoskeleton is crustacean; option C.
All organisms have either an endoskeleton or an exoskeleton is False because plants do not have either an endoskeleton or an exoskeleton.
What are the structures for support found in plants?
Plants unlike animals do not have skeletons that provide shape and support to them.
Instead, plants have sclerenchyma cell walls which are composed of lignin and extra cellulose. These provide support and shape to plants.
Ligaments are structures found in joints and other parts of the body that provide support to bones, joints, organs, and other structures.
Ligaments in the body include;
The anterior cruciate ligament (ACL)The ulnar collateral ligaments The anterior talofibular ligamentExoskeletons are found in small animals and insects. For example, crustaceans have an exoskeleton.
Other larger organisms such as mammals. have an endoskeleton.
Learn more about exoskeleton and endoskeleton at: https://brainly.com/question/2661307
#SPJ1

