Purpose: Deteining phosphate in the soil using a method which can be carried out in the field to obtain results on the spot.
Procedure:
Weight out 5 g of soil samples (5) using small scoop or spatula. For reproducibility, the soil samples should be about the same volume.
Label 15 mL Falcon tubes with caps, and add5 ml of deionized water.
Transfer the soil samples to the 15 mL falcon tubes that contain 5 mL of deionized water.
Cape the sample tubes and invert 10 times with shaking and allow to settle for 15 minutes.
Transfer liquid in the sample tube along some soil to a 1oml syringe which is subsequently filler with a filter (B-D™ Disposable Syringes, Luer-Lock Tips, 10 mL, # 14823 2A; Cole-Paer Nylon Syringe Filters, 0.45 μm, 25 mm diameter; Item# UX-02915-14; equivalent syringes and filters can be used).
Inject soil extracted via filter into a nother labeled 15 ml falcon tube.
Label reaction microfuge tubes (1-5).
Set up 0.5ml of a reaction mixture containing:
200 mM HEPES
pH 7.6
20 mM MgCl2
containing 80 nmol MESG
1 unit of recombinant PNP (NECi recombinant PNP1, 1 unit = 1 μmol phosphate consumed per min, see Nitrate.com; or equivalent)
Allow it to mix on filed temperature.
Transfer 500 μL sample of each soil extracted by micropipette to labeled microfuge tubes containing reaction mixture.
Cape the tube and invert 3 times.
Incubate the tubes for about 10 minutes at filed temperature.
Transfer the contents of the reaction tubes to methylacrylate (PMMA) disposable cuvettes (UV-Cuvette Disposable Photometer Cuvette, VWR catalog No. 47727-024, or equivalent).
Set absorbance at 360 nm for each soil sample.
Use deionized water as a blank for a portable photometer.
Compare the absorbance of each sample to the standard curve prepared in advance with certified KH2PO4 standard 1000 ppm.
Use linear regression equation of the standard curve to calculate and record the inorganic content of phosphate.
Results can be reported ppm phosphate per volume of soil sampled (i.e., volume of the scoop used to sample the soil). The results may also be reported as phosphorus, by simply dividing the phosphate results by 3.1 to obtain ppm phosphorus (mg PO4–P/L) 97/31=3.1.
For greater precision, the soil should be dried to constant weight and 1 gm of dry soil extracted with 5 mL of deionized water.
Answers
Phosphate determination in the soil using field methods requires a procedure that can give immediate results. The procedure that is described below is one such example.
It involves weighing out 5 g of soil samples, labeling 15 mL Falcon tubes with caps, and adding 5 ml of deionized water to the labeled tubes. The soil samples are then transferred to the labeled 15 mL Falcon tubes containing 5 mL of deionized water. The sample tubes are capped and shaken and allowed to settle for 15 minutes. After the 15 minutes have passed, the liquid in the sample tube is transferred to a 10 mL syringe that is then filled with a filter.
The sample of each soil extracted through the microfuge tube is transferred to the labeled microfuge tubes containing the reaction mixture using a micropipette. The tube is capped and shaken. The tubes are then incubated for about 10 minutes at field temperature. After the 10 minutes, the contents of the reaction tubes are transferred to methyl acrylate (PMMA) disposable cuvettes, and the absorbance is set at 360 nm for each soil sample. Deionized water is used as a blank for a portable photometer.
To know more about Phosphate visit:
brainly.com/question/24923664
#SPJ11
Related Questions
What are the 4 types of weathering?
Answers
Answer: The four main types of weathering is I think, Chemical, Physical, Biological, and Mechanical weathering. But if you're talking about main agents then it will have to be water, ice, air, and temperature.
Explanation:
Hope this helps! Tried my best.
A car travels with a force of 10,000N and has a mass of 500 kg. What is the acceleration of the car?
Answers
Explanation:
we have,
force(f)=10,000N
mass(m)=500kg
now,
acceleration=f×m
=10,000×500
=5000000m/s²
6
What is the density of a substance that has a mass of 2.0 g, and when placed in a graduated cylinder
the volume changed from 70 mL to 75 mL?
A 2.5 g/mL
B 7.0 g/mL
C 10. g/mL
D 0.40 g/mL
Answers
The density of the substance having a mass of 2.0 g is 0.4 g/mL (Option D)
How do I determine the density of the substance?First, we shall obtain the volume of the substance. This can be obtained as follow:
Volume of water = 70 mL Volume of water + substance = 75 mL Volume of substance =?Volume of substance = (Volume of water + substance) - (Volume of water)
Volume of substance = 75 - 70
Volume of substance = 5 mL
Finally, we shall determine the density of the substance. This is illustrated below:
Mass of substance = 2.0 gVolume of substance = 5 mLDensity of substance = ?Density = mass / volume
Density of substance = 2 / 5
Density of substance = 0.4 g/mL
Thus, the density is 0.4 g/mL (Option D)
Learn more about density:
https://brainly.com/question/952755
#SPJ1
What type of glass has been exposed to high temperatures, so that when it breaks, it shatters into tiny pebble-like fragments that are less dangerous?
Answers
The type of glass that has been exposed to high temperatures and shatters into tiny pebble-like fragments when it breaks is called tempered glass.
Tempered glass is a type of safety glass that has been treated with heat and chemicals to make it stronger and more durable than regular glass. When it breaks, it shatters into small, rounded fragments that are less likely to cause injury than the sharp shards produced by regular glass. Tempered glass is commonly used in applications where safety is a concern, such as car windows, shower doors, and storefront windows. It is also used in the construction of buildings, furniture, and appliances.
To know more about temperature
https://brainly.com/question/11464844
#SPJ4
Draw the alkyl bromide(s) you would use in a malonic ester synthesis of ethyl 2-methyl-4-pentenoate
Answers
The structure of the alkyl bromides used in a malonic ester synthesis of ethyl 2-methyl-4-pentenoate are as drawn in the attached image.
Ethyl 2-methyl-4-pentenoate by Malonic ester synthesis.The malonic ester synthesis is a chemical reaction characterized by the alkylation of diethyl malonate or a similar ester of malonic acid at the carbon alpha (directly adjacent) to both carbonyl groups, and subsequently converted to a substituted acetic acid.
Hence, it follows from the structure of Ethyl 2-methyl-4-pentenoate that the alkyl bromides used are Ethyl bromide and methyl bromide.
Read more on Malonic ester synthesis;
https://brainly.com/question/17237043
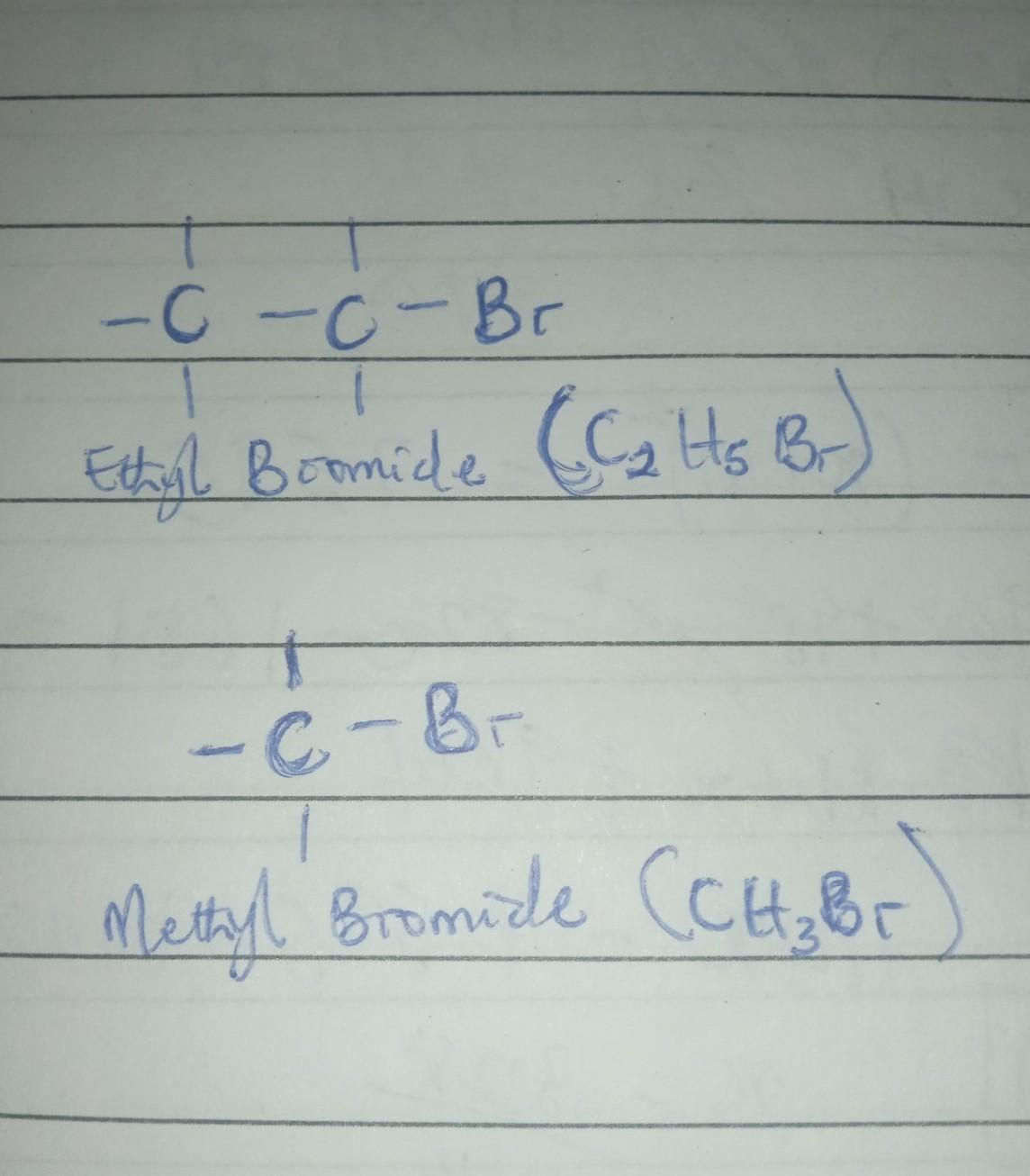
The structure of alkyl bromide that would be used in a malonic ester synthesis of ethyl 2-methyl-4-pentenoate are ethyl bromide and methyl bromide.
What is alkyl bromide?
When halogens such as Br, Cl, I, attched to the sp3 carbon atom of alkyl group, they called alkyl halides.
Here, bromide is attached to an alkyl group.
Ethyl bromide and methyl bromide are the alkyl halides used in synthesis of ethyl 2-methyl-4-pentenoate
Thus, ethyl bromide and methyl bromide are the alkyl bromides.
Learn more about alkyl bromide
https://brainly.com/question/14126879
#SPJ4


What's the fomular for this?

Answers
Answer:
1000 km/s is the formula for density of liquid
what is the percent composition of hydrogen in beryllium hydride(BeH2) if 69.6g of beryllium (Be) react with 15.6 g of hydrogen to produce 85.2 g of BeH2
Answers
The percent composition of hydrogen is 18.3%.
What is percent composition?The term percent composition refers to the percentage of a particular component in a compound. It is contained as the ratio of the mass of that component to the total mass multiplied by 100.
From the law of conservation of mass, total mass of beryllium hydride(BeH2) = 85.2 g
Mass of hydrogen = 15.6 g
Percent composition of hydrogen = 15.6 g/ 85.2 g × 100/1 = 18.3%
Learn more about percent composition: https://brainly.com/question/12247957
Describe what occurs when a metal is placed into an acid and the equation and what is occurring within it
Answers
When a metal is placed into an acid, a chemical reaction called a redox reaction occurs. In this process, the metal loses electrons (oxidation) and the acid gains electrons (reduction). This results in the formation of a salt and hydrogen gas is released. The general equation for this reaction is:
Metal + Acid → Salt + Hydrogen gas
For example, when zinc is placed into hydrochloric acid, the reaction is:
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
In this reaction, zinc is oxidized to form zinc chloride (ZnCl₂) while hydrogen ions (H⁺) in the hydrochloric acid are reduced to form hydrogen gas (H₂).
The release of hydrogen gas can be observed as bubbles forming in the solution. This process demonstrates the reactivity of metals with acids, which depends on the metal's position in the reactivity series.
To know more about redox reaction refer here:
https://brainly.com/question/13293425#
#SPJ11
Mg(s) + HCl(aq) −→ MgCl2(aq) + H2(g).
What mass of HCl is consumed by the reaction of 2.28 mol of magnesium?
Answer in units of g
Answers
166 g HCl
General Formulas and Concepts:Math
Pre-Algebra
Order of Operations: BPEMDAS
BracketsParenthesisExponentsMultiplicationDivisionAdditionSubtractionLeft to RightChemistry
Stoichiometry
Reading a Periodic TableBalancing EquationsUsing Dimensional AnalysisExplanation:Step 1: Define
[RxN - Unbalanced] Mg (s) + HCl (aq) → MgCl₂ (aq) + H₂ (g)
[RxN - Balanced] Mg (s) + 2HCl (aq) → MgCl₂ (aq) + H₂ (g)
[Given] 2.28 mol Mg (s)
Step 2: Identify Conversions
[RxN] 1 mol Mg = 2 mol HCl
Molar Mass of H - 1.01 g/mol
Molar Mass of Cl - 35.45 g/mol
Molar Mass of HCl - 1.01 + 35.45 = 36.46 g/mol
Step 3: Stoichiometry
Set up: \(\displaystyle 2.28 \ mol \ Mg(\frac{2 \ mol \ HCl}{1 \ mol \ Mg})(\frac{36.46 \ g \ HCl}{1 \ mol \ HCl})\)Multiply: \(\displaystyle 166.258 \ g \ HCl\)Step 4: Check
Follow sig fig rules and round. We are given 3 sig figs.
166.258 g HCl ≈ 166 g HCl
how many dots would be found in the lewis dot structure for the compound c2h3cl3?
Answers
The number of dots would be found in the Lewis dot structure for the compound \(C_{2} H_{3}Cl_{3}\) is 32.
To determine the number of dots in the Lewis dot structure for the compound \(C_{2} H_{3} Cl_{3}\) , we first need to know the structure. In the Lewis dot structure, each hydrogen atom has two dots representing two valence electrons and each chlorine atom has six dots representing six valence electrons. The carbon atoms each have four dots representing four valence electrons on their own atoms, and one additional dot on the double bond between them. Therefore, the total number of dots in the Lewis dot structure for the compound \(C_{2} H_{3} Cl_{3}\) is:
(2 x 4) + (3 x 2) + (3 x 6) = 8 + 6 + 18 = 32
Learn more about Lewis dot structure here :
https://brainly.com/question/20300458
#SPJ11
There would be 32 dots in the Lewis dot structure for the compound \(C_{2}H_{3}Cl_{3}\).
How to determine the number of dots in a compound?To determine the number of dots in the Lewis dot structure for the compound \(C_{2}H_{3}Cl_{3}\)., we need to calculate the total number of valence electrons for each element in the compound.
1. Identify the number of valence electrons for each element:
- Carbon (C) has 4 valence electrons.
- Hydrogen (H) has 1 valence electron.
- Chlorine (Cl) has 7 valence electrons.
2. Calculate the total number of valence electrons in the compound:
- There are 2 carbon atoms, so 2 * 4 = 8 valence electrons for carbon.
- There are 3 hydrogen atoms, so 3 * 1 = 3 valence electrons for hydrogen.
- There are 3 chlorine atoms, so 3 * 7 = 21 valence electrons for chlorine.
3. Add up the total number of valence electrons:
- 8 (from carbon) + 3 (from hydrogen) + 21 (from chlorine) = 32 valence electrons.
To know more about Lewis Structures:
https://brainly.com/question/12792895
#SPJ11
help please bjfkboweugfowbs

Answers
Answer:
B is correct answer because The enthalpy is negative so this means the reaction produces heat and the reaction is exothermic
Hamood backwards is hamood
Answers
Answer:
dang bro, thats deep
Explanation:
so deep imma cry
How will designing more efficient stoves help conserve fuel?
Answers
Answer:
it would do wonders for the health of those using them and, by extracting energy from the fuel more efficiently, would be more environmentally sustainable too.
what is the total number of joules of heat energy needed to raise the temperature of 10 grams of water from 20 c to 30 c
Answers
The total number of joules of heat energy needed to raise the temperature of 10 grams of water from 20°C to 30°C is 418.4 J. The specific heat capacity of water is 4.184 J/g·°C.
To find the total heat energy needed, we can use the formula:
Q = m·c·ΔT
where:
Q = heat energy (in Joules)
m = mass of the water (in grams)
c = specific heat capacity of water (4.184 J/g·°C)
ΔT = change in temperature (in °C)
Substituting the values given, we get:
Q = 10 g × 4.184 J/g·°C × (30°C - 20°C)
Q = 418.4 J
Therefore, the total number of joules of heat energy needed to raise the temperature of 10 grams of water from 20°C to 30°C is 418.4 J.
Learn more about heat energy
https://brainly.com/question/29210982
#SPJ4
As temperature increase and the volume stays constant, how will the pressure change
Answers
Answer:
There will be more collisions and so a greater pressure. The number of particles is proportional to pressure, if the volume of the container and the temperature remain constant. ... Volume is inversely proportional to pressure, if the number of particles and the temperature are constant.
the tendency for water to move toward greater solute concentration is an example of
Answers
Osmosis is the movement of water molecules from an area of higher water concentration to an area of lower water concentration across a semipermeable membrane.
This movement of water occurs because of the tendency for water to move towards a higher solute concentration in order to achieve equilibrium. Solutes are particles that are dissolved in water and they decrease the amount of available water molecules. Therefore, when solute concentration is higher on one side of a semipermeable membrane, the water molecules move towards the solute in order to balance out the concentration levels on both sides. This process is crucial for many biological processes such as maintaining proper cell function and the absorption of nutrients in plants.
Osmosis is the process in which water molecules move across a selectively permeable membrane from an area of lower solute concentration to an area of higher solute concentration. This movement continues until an equilibrium is reached, where the solute concentrations are equal on both sides of the membrane.
To know more about molecules visit:
https://brainly.com/question/30465503
#SPJ11
write electronic configuration of chlorine in its ionic state?
Answers
Answer:
1s2 2s2 2p6 3s2 3p6
Explanation:
Chlorine is a groups 17 element. The halogens for ions by accepting one electron to form univalent negative ions.
Since chlorine normally contains seventeen electrons, the chloride ion consists of eighteen electrons.
Hence the electronic configuration of chlorine ion is; 1s2 2s2 2p6 3s2 3p6.
A student converted 400 centimeters into meters. Which of the following shows the student's answer with the correct number of significant digits?
Answers
Answer:
4 meters
Explanation:
100 centimeters to meters, so move the decimal 2 spaces left.
400 becomes 4.00
A McLeod gauge measures low gas pressures by com- pressing a known volume of the gas at constant temperature. If 345 cm of gas is compressed to a volume of 0.0457 cm³ under a pressure of 2.51 kPa, what was the original gas pressure?
Answers
The pressure of a gas is the force that the gas exerts on the walls of its container.
How do you determine the initial pressure of a gas?Let’s start with the ideal gas law, PV = nRT. In this equation, ‘P’ represents atmospheric pressure, ‘V’ represents volume in liters, ‘n’ represents the number of particles in moles, ‘T’ represents temperature in Kelvin, and ‘R’ represents the ideal gas constant (0.0821 liter atmospheres per moles Kelvin). The force that a gas exerts on the walls of its container is defined as its pressure.
When you blow air into a balloon, it expands because the pressure of air molecules on the interior of the balloon is greater than on the outside. Pressure is a characteristic that governs the direction of mass flow.
To learn more about pressure of a gas to refer:
https://brainly.com/question/15110960
#SPJ1
Dmitri mendeleev contribution to the periodic table.
Answers
In 1871, a Russian Chemist, Dimitri Mendeleev, gave a useful scheme for classification of elements. He presented the first regular periodic table in which elements of similar chemical properties were arranged in eight vertical columns called groups. The horizontal rows of table were called periods. He arranged elements in ascending order of their atomic masses and found that elements having similar chemical properties appeared at regular intervals. This observation was called Periodic Law.
What should you do if you do not observe any difference in the TLC after 20 minutes? What does this say about the reaction or the analytical method?
What is occurring chemically with the sodium bisulfite? What is the orange color and why does it go away with the sodium bisulfite treatment?
What if, upon cooling, no crystals form? What can you conclude about this observation? What should you do in this case?
Answers
If one does not observe any difference in the TLC after 20 minutes, it shows that the reaction was not carried out or did not take place. In such a case, one should repeat the reaction under optimal conditions.
In such a case, you should consider rechecking the reaction or the analytical technique used. This situation suggests that the reaction may be unsuccessful due to a technical issue such as failure to provide necessary conditions for the reaction to occur. It may also imply that the reaction being analyzed did not undergo any significant transformation, hence no difference was observed.
One can solve this problem by changing the solvent and considering the pH of the solution to provide optimal conditions for the reaction to occur. A more sensitive analytical method could also be employed to detect small changes or differences. The primary cause of the orange color is impurities present in the product, which are subsequently reduced to form the final product through sodium bisulfite treatment. When cooled, if no crystals form, it indicates that the product did not form, and the reaction did not take place.
This can result from an incorrect ratio of reactants, the purity of reagents, or incorrect reaction conditions. In such a case, one should repeat the reaction under optimal conditions.
To know more about optimal conditions visit:
https://brainly.com/question/30547120
#SPJ11
Imagine that the human population continues to increase, but we don't monitor or implement strategies to minimize our impact on the blue crab population.
Construct an argument supported by evidence for how increases in human populations impact the consumption of natural resources. In your answer, be
sure to include:
• the relationship between human populations and the consumption of resources
• a prediction of how the blue crab population would change with an increasing human population
evidence from the resources explored during the lesson
scientific reasoning
Answers
Answer:
The Explanation
Explanation:
:i imagine less blue crabs ngl
a soup in a container was forgotten in the refrigerator and shows contamination. the contaminants are probably which of the following? group of answer choices thermophiles acidophiles mesophiles psychrophiles alkaliphiles
Answers
A soup in a container was forgotten in the refrigerator and shows contamination. the contaminants are probably acidophiles.
The word "contamination" in chemistry often refers to a single component, but in some specialised domains, it can also refer to chemical combinations, even down to the amount of cellular components. Every chemical has certain impurities in it. If the impure chemical produces extra chemical reactions when combined with additional substances or mixes, contamination may be seen or not, and it may also become a problem. A soup in a container was forgotten in the refrigerator and shows contamination. The contaminants are probably acidophiles.
Therefore, the contaminants are probably acidophiles.
To learn more about contamination, here:
https://brainly.com/question/24324754
#SPJ4
What is the solubility of calcium sulfate (CaSO4) in 0. 30 M aqueous sodium sulfate (Na2SO4)? (Ksp of calcium sulfate = 2. 0 x 10^-5
Answers
The solubility product constant (Ksp) of calcium sulfate (CaSO4) and the idea of ion product may be used to determine the solubility of calcium sulfate (CaSO4) in 0.30 M aqueous sodium sulfate.
(Na2SO4). The calcium ion (Ca2+) concentration in the solution. may be estimated as follows: [Ca2+][SO42-] = Ksp [Ca2+][SO42-] = 2.0 x 10^-5 When there is sodium sulfate present, part of the sulfate ions will originate from the sodium sulfate rather than the calcium sulfate. The total sulfate ion concentration (SO42-) in the solution may be determined as follows [SO42-] = [Na2SO4] + [CaSO4] + x = 0.30 M (where x is the concentration of CaSO4) When we enter the values into the Ksp expression, we get: 2.0 x 10^-5 = [Ca2+] [0.30 M + x] x [0.30 M + x] = [Ca2+] x [0.30 M + x] [Ca2+] = (2.0 x 10^-5) / (0.30 M + x) We may substitute [Ca2+] in the Ksp equation since [Ca2+] = [CaSO4]: ([CaSO4])2 / (0.30 M + [CaSO4]) = 2.0 x 10-5 This equation may be solved for [CaSO4,] which yields the calcium sulfate solubility in 0.30 M aqueous sodium sulfate solution.
learn more about solubility here:
https://brainly.com/question/29661360
#SPJ4
(0)
Calculate the standard enthalpy of reaction for a system in which increasing the temperature by 15 K reduces the equilibrium constant by half, relative to its value at 310 K.
Give your answer in kJ/mol
_________________________
Answers
The standard enthalpy of reaction for a system in which increasing the temperature by 15 K reduces the equilibrium constant by half, relative to its value at 310 K is -0.956 kJ/mol.
Given: ΔT = 15 K and ∆K/K = 1/2
Temperature is directly proportional to equilibrium constant K,
So, ∆T/T = ∆K/K
This can be written as ∆K/K = ΔH°/R × (1/T2 − 1/T1)
On solving this equation, we getΔH° = −2.303 × R × ΔK/K × T2T2 = 310 + 15 = 325 K∆K/K = 1/2∆H° = −2.303 × 8.314 J mol−1 K−1 × 1/2 × 325 K∆H° = −955.7 J mol−1= −0.956 kJ/mol
Therefore, the standard enthalpy of reaction for a system in which increasing the temperature by 15 K reduces the equilibrium constant by half, relative to its value at 310 K is -0.956 kJ/mol.
Learn more about enthalpy with the given link,
https://brainly.com/question/14047927
#SPJ11
ordinary household bleach is an aqueous solution of sodium hypochlorite. what is the molarity of a bleach solution that contains 27.5 g of sodium hypochlorite in a total volume of 424 ml? m
Answers
The molarity of a bleach solution that contains 27.5 g of sodium hypochlorite in a total volume of 424 ml is 0.87 M.
Mass of sodium hypochlorite(NaClO) in solution = 27.5 g
The volume of solution = 424 ml
To calculate the molarity of a solution, we can use the formula:
Molarity = (Number of moles of solute) / (Volume of solution in liters)
Using the above formula to find the molarity of the bleach solution.
Number of moles of NaClO = (mass of NaClO) / (Molar mass of NaClO)
The molar mass of NaClO = (23+35.5+16) = 74.5 g/mol
Hence, the number of moles of NaClO = 27.5 / 74.5 = 0.369 moles
Volume of solution in liters = 424 ml = 424 / 1000 = 0.424 litres
Hence, Molarity of bleach solution = 0.369 / 0.424 = 0.87 M
Therefore, the molarity of the bleach solution is 0.87 M.
To know more about sodium hypochlorite, refer here:
https://brainly.com/question/15312359#
#SPJ11
When copper is heated in air, the solid product that is formed has a greater mass than the original copper. When calcium carbonate is heated in air, the solid product that is formed has less mass than the original
calcium carbonate. Why is the mass of the solid product greater in one reaction but less in the other reaction?
Answers
Answer:
When heated, oxygen reacts with copper to form copper oxide. If this reaction occurs in a sealed container, will the mass of the container and everything in it increase, decrease, or stay the same and why? The mass will increase because a new kind of molecule is formed.
Explanation:
As above, carbonates decompose upon heating to give the metal oxide and carbon dioxide. The mass is lost as carbon dioxide.
To solve this we must be knowing each and every concept related to mass. Therefore, Carbon dioxide is released as the mass is depleted when carbonates are heated.
What is mass?Mass defines the quantity of a substance. It is measured in gram or kilogram. Average mass is the mass of atoms of an element that are isotopes.
When copper was heated in the air, the resulting solid product has a larger mass than the initial copper. When calcium carbonate was heated in air, the resulting solid product has much less mass than the initial calcium carbonate. When carbonates are heated, they breakdown into metal oxides and carbon dioxide. Carbon dioxide is released as the mass is depleted.
Therefore, Carbon dioxide is released as the mass is depleted.
To learn more about mass, here:
https://brainly.com/question/28704035
#SPJ2
What is the rate of production of product X if its concentration increases from 3.40x10-3 mol/L to 5.70x10-3 mol/L in 228.9 seconds.
Answers
The rate of production of product X is approximately 9.13 x \(10^{-6}\) mol/(L·s), indicating an increase in concentration by that amount per second.
To determine the rate of production of product X, we need to calculate the change in concentration of X divided by the change in time. The change in concentration is obtained by subtracting the initial concentration (3.40 x \(10^{-3}\) mol/L) from the final concentration (5.70x\(10^{-3}\)mol/L), resulting in a concentration change of 2.30 x \(10^{-3}\) mol/L.
Next, we divide the concentration change by the change in time, which is given as 228.9 seconds. Dividing the concentration change of 2.30 x \(10^{-3}\) mol/L by the time of 228.9 seconds gives us the rate of production of product X.
The rate of production of X is calculated as (5.70x\(10^{-3}\) mol/L - 3.40x10-3 mol/L) / 228.9 seconds, which simplifies to approximately 9.13x\(10^{-6}\)mol/(L·s). This indicates that for every second, the concentration of product X increases by 9.13x10-6 mol/L.
You can learn more about rate of production at
https://brainly.com/question/29886282
#SPJ11
Can anyone help please?

Answers
Answer:
cytsdfywty ++=
Explanation:
V-pneumonoultramicroscopicsilicovolcanoconiosis
Which of these molecules should be able to pass through a gap junction?
Amyloid
Hexokinase
Glucose
Potassium ion
Answers
The molecule that should be able to pass through a gap junction is the potassium ion (Option D).
What are gap junctions?Gap junctions are protein channels that allow direct intercellular communication between adjacent cells. They allow small molecules such as glucose and ions such as potassium ions to pass through. Gap junctions are critical in coordinating cellular activities such as contraction in heart muscle cells, secretion of hormones, and the release of neurotransmitters by neurons. Therefore, gap junctions form channels that allow small molecules such as glucose and ions such as potassium ions to pass through.
Thus, the correct option is D.
Learn more about gap junction: https://brainly.com/question/30640667
#SPJ11