Provide a first law analysis for each of the following cases: (a) When a bicycle tire is inflated with a hand pump, the temperature inside rises. You can feel the waing effect at the valve stem. (b) Artificial snow is made by quickly releasing a mixture of compressed air and water vapor at about 20 atm from a snow-making machine to the surroundings.
Answers
(a) When a bicycle tire is pumped by hand, the internal temperature increases due to the compression process. As the air inside the tire is compressed, its pressure rises while the volume decreases. According to the first law of thermodynamics, this compression work increases the internal energy of the gas, resulting in a temperature rise. The valve stem, where air is introduced, experiences a warming effect as a consequence of this increased internal energy.
(b) In the case of producing artificial snow, a snow-making machine releases a mixture of compressed air and water vapor into the surroundings. This rapid release causes the gas to expand quickly, performing work on the surroundings. As per the first law of thermodynamics, this expansion work reduces the internal energy of the gas, leading to a decrease in temperature. Consequently, the rapid expansion of compressed air and water vapor induces a cooling effect, aiding the formation of artificial snow.
You can learn more about temperature at
https://brainly.com/question/27944554
#SPJ11
Related Questions
a 64g sample of germanium-66 is left undisturbed for 12.5 hours. at the end of that period, only 2.0g remain. what is the half-life of this material?
Answers
A 64g sample of germanium-66 is left undisturbed for 12.5 hours. at the end of that period, only 2.0g remain. The half-life of germanium-66 is approximately 2.5 hours.
To find the half-life of germanium-66, we can use the formula N(t) = N0 * (1/2)^(t/T), where N(t) is the remaining amount at time t, N0 is the initial amount, T is the half-life, and t is the time elapsed. We have N(t) = 2g, N0 = 64g, and t = 12.5 hours. Plugging these values into the formula, we get:
2 = 64 * (1/2)^(12.5/T)
Now, we need to solve for T. First, divide both sides by 64:
(2/64) = (1/2)^(12.5/T)
Simplify the left side:
1/32 = (1/2)^(12.5/T)
Next, take the logarithm of both sides:
log2(1/32) = (12.5/T) * log2(1/2)
Solve for T:
T = 12.5 / log2(32)
T ≈ 2.5 hours
Therefore, the half-life of germanium-66 is approximately 2.5 hours.
Learn more about half-life here:
https://brainly.com/question/24710827
#SPJ11
Predict which substance in each pair has the highest viscosity:1. octane (ch3ch2ch2ch2ch2ch2ch2ch3) or 1-octanol (ch3ch2ch2ch2ch2ch2ch2ch2oh) 2. butane or isobutane
Answers
The substance with the highest viscosity in each pair can be predicted by comparing the molecular structures of the substances. Viscosity is a measure of a fluid's resistance to flow, and is affected by the strength of intermolecular forces between the molecules of the substance.
1. Octane (CH3CH2CH2CH2CH2CH2CH2CH3) and 1-octanol (CH3CH2CH2CH2CH2CH2CH2CH2OH): The molecular structure of 1-octanol includes an -OH functional group, which can form hydrogen bonds with other 1-octanol molecules. This results in stronger intermolecular forces and a higher viscosity compared to octane, which only has van der Waals forces between its molecules. Therefore, 1-octanol has a higher viscosity than octane.
2. Butane and isobutane: Both butane and isobutane are hydrocarbons with the same molecular formula (C4H10), but they have different molecular structures. Butane is a straight-chain alkane, while isobutane is a branched-chain alkane. The straight-chain structure of butane allows for more contact between the molecules, resulting in stronger van der Waals forces and a higher viscosity compared to isobutane. Therefore, butane has a higher viscosity than isobutane.
In conclusion, the substance with the highest viscosity in each pair is 1-octanol and butane.
To know more about viscosity refer here:
https://brainly.com/question/30577668
#SPJ11
Which process has the greatest potential for genetic variability?
A.
cell differentiation
B.
interkinesis
C.
meiosis
D.
mitosis
Answers
what type of substances are required for the production of illicit drugs?
Answers
The substances required for the production of illicit drugs vary depending on the type of drug being produced. For example, methamphetamine production typically involves the use of chemicals such as pseudoephedrine, anhydrous ammonia, and lithium, while cocaine production requires coca leaves and other chemicals like hydrochloric acid.
Other illicit drugs like heroin, ecstasy, and LSD also require specific substances for production. It is important to note that the production of illicit drugs is illegal and poses significant health and safety risks.
to know more about substances intake pls visit:
https://brainly.com/question/7482280
#SPJ11
When two or more simple machines are combined they form a(n) ____.
A. Compound machine
B. Complex machine
C.intricate machine
D.inefficient machine
Answers
Answer:
A
Explanation:
A compound machine is a combination of two or more simple machines.
Compared to compounds with network structures, compounds arranged in molecules (covalent bonds) have ____________ melting points.
Answers
Explanation:
.... have lower melting points.
a ____________________ is a stream from which water is moving downward to the water table?
Answers
A "percolating stream" is a stream from which water is moving downward to the water table.
A percolating stream generally refers to a type of stream or water flow that occurs when water moves downward through permeable materials such as soil, sand, or rock, gradually infiltrating into the ground. The water follows a vertical or nearly vertical path, seeping through the interconnected spaces and pores within the subsurface layers.
This movement is often driven by gravity, as water percolates or filters through the porous medium. Percolating streams contribute to groundwater recharge, replenishing underground water reservoirs and influencing the overall hydrological cycle.
Learn more about percolating stream from the link given below.
https://brainly.com/question/30410369
#SPJ4
How many grams of hydrogen peroxide are needed to produce 90. 0 g of water?
2H202 (1) ► 2H20() + O2(g)
Answers
Put the numbers into your calculator to find the answer! 90 x 32, then divide by 18.02 x 2. Estimation can tell me that the answer is 79.9, or 80!
What is the potential energy of the reactants?
a.) 20kJ
b.)40kJ
c.)60kJ
d.)80kJ
e.)100kJ
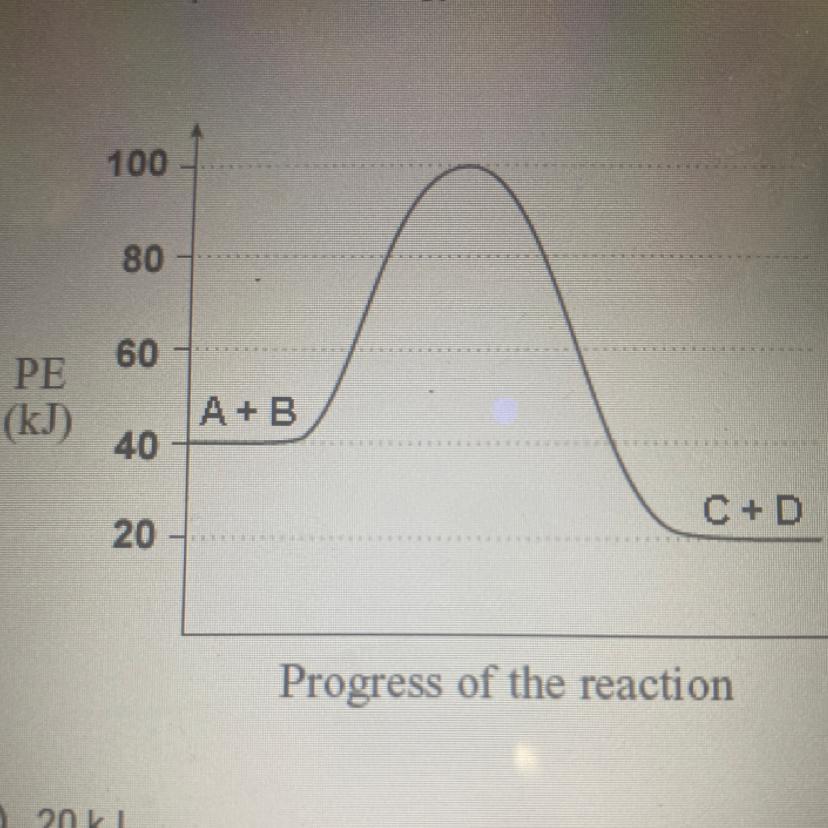
Answers
The given plot represents the change in energy of the system with respect to the progress of the reaction for an exothermic reaction. The potential energy of the reactants is 40 kJ. The correct option is B.
What is an exothermic reaction?The chemical reactions which proceed with the evolution of heat energy are called exothermic reactions. In an exothermic reaction the system loses heat to the surroundings. So 'q' and ΔH will be negative.
Since heat is given out in exothermic reactions, the enthalpy of the products will be less than that of the reactants. Here the potential energy of the reactants is 40 kJ.
Thus the correct option is B.
To know more about exothermic reaction, visit;
https://brainly.com/question/28546817
#SPJ9
Ca2+ ions (essential for contraction) are stored in the
a. sarcoplasm
b. sarcolemma
c. sarcoplasmic reticulum
d. T-tubules
Answers
The correct answer is c. sarcoplasmic reticulum. Ca2+ ions, which are essential for muscle contraction, are stored in the sarcoplasmic reticulum (SR) of muscle cells.
The sarcoplasmic reticulum is a specialized network of membranous sacs within muscle fibers, specifically designed for the storage and release of calcium ions during muscle contraction.
When a muscle is stimulated, an action potential triggers the release of stored Ca2+ ions from the sarcoplasmic reticulum into the sarcoplasm, the cytoplasm of muscle cells. The influx of Ca2+ ions into the sarcoplasm initiates a series of events leading to muscle contraction.
The sarcoplasm refers to the cytoplasm of muscle cells, the sarcolemma is the plasma membrane of muscle cells, and T-tubules are invaginations of the sarcolemma that help transmit the action potential deep into the muscle fiber.
Therefore, the correct location where Ca2+ ions are stored for muscle contraction is the sarcoplasmic reticulum (c).
To learn more about contraction
https://brainly.com/question/1166774
#SPJ11
Which method would provide the best synthesis of ethyl isopropyl ether? b) CH3CH2ONa + (CH3)2CHBr H2S04, 140 °C H2SO4, 180 °C d) CH3CH2OH +(CH3)2CHOH e) CH3CH2ONa +(CH3)2CHOH
Answers
CH\(_{3}\)CH\(_{2}\)ONa + (CH\(_{3}\))\(_{2}\) CHBr H\(_{2}\)S0\(_{4}\), 140 °C would provide the best synthesis of ethyl isopropyl ether. The correct answer is option b).
This is because the method involves the use of sodium ethoxide (CH\(_{3}\)CH\(_{2}\)ONa) and isopropyl bromide ((CH\(_{3}\))\(_{2}\) CHBr) in the presence of sulfuric acid. This process is known as the Williamson ether synthesis and is an effective method for the preparation of ethers.
The other options are not suitable for the synthesis of ethyl isopropyl ether. Option d) involves the use of ethanol and isopropyl alcohol which would not react to form the desired ether product. Option e) involves the use of sodium ethoxide and isopropyl alcohol which would result in the formation of isopropyl ethyl ether instead of ethyl isopropyl ether.
Overall, the Williamson ether synthesis is the most appropriate method for the synthesis of ethyl isopropyl ether. The correct answer is option b.
You can learn more about ethyl isopropyl at
https://brainly.com/question/31044163
#SPJ11
What is the carbon nucleophile which attacks molecular bromine in the acid-catalyzed α-bromination of a ketone?
Answers
The carbon nucleophile which attacks molecular bromine in the acid-catalyzed α-bromination of a ketone an acetylide. The Organometallic reagents like those used in the Grignard, Blaise, Reformatsky, and Barbier reactions as well as reactions involving the organolithium reagents and acetylides are frequently used as carbon nucleophiles.
These chemicals are frequently employed in nucleophilic additions. Compounds or intermediates that contain an electron-rich carbon atom are known as carbon-centered nucleophiles because they have the ability to donate an electron pair from that carbon atom to an electrophile. When writing resonance structures, the strong negative nature of a metal-bonded the carbon is apparent.
To learn more about carbon, click here.
https://brainly.com/question/13719781
#SPJ4
based on the h and s values for a given chemical reaction, it is possible to predict whether the reaction is spontaneous or not at various temperatures. which one of the following statements has the most correct answers? question 38 options: (a) if h and s are both positive, the reaction will always be spontaneous (b) if h and s are both positive, the reaction will be spontaneous at a high enough temperature (c) if h is negative and s is positive, the reaction will always be spontaneous (d) if h and s are both negative, the reaction will always be spontaneous (e) both (b) and (c) g
Answers
The correct answer is (b) if h and s are both positive, the reaction will be spontaneous at a high enough temperature.
The spontaneity of a chemical reaction is determined by the change in Gibbs free energy (ΔG) of the system. The relationship between enthalpy (ΔH), entropy (ΔS), and Gibbs free energy (ΔG) is given by the equation:
ΔG = ΔH - TΔS,
where T represents the temperature in Kelvin.
For a spontaneous reaction, ΔG must be negative. From the equation, we can observe that if both ΔH and ΔS are positive, the reaction can still be spontaneous if the temperature is high enough. As the temperature increases, the negative term TΔS becomes more significant and can overcome the positive term ΔH, resulting in a negative ΔG.
Therefore, option (b) is the most correct statement, stating that if both h and s are positive, the reaction will be spontaneous at a high enough temperature.
Learn more about spontaneous reaction
brainly.com/question/14312067
#SPJ4
what is vapor density?
Answers
Vapor density is defined as the amount of weight of a gas or vapor in comparison to air.
The relative weight of a gas or vapor in comparison to air, which has an arbitrary value of one, is defined as vapor density. If a gas's vapor density is less than one, it will rise in air. When the vapor density exceeds one, the gas will normally sink in air.
Vapor density is only a broad concept used to estimate where vapors might be discovered when released. This physical parameter, however, is not absolute and can be influenced by:
Air currentsTemperatureMaterial released from its container HumidityDew pointAerosolsTo learn more about vapor density, click here:
https://brainly.com/question/28187501
#SPJ4
Calculate the equilibrium concentrations of n2o4 and no2 after the extra 1. 00 mol no2 is added to 1. 00 l of solution.
Answers
The equilibrium concentrations of N2O₄ and NO₂ after the addition of 1.00 mol NO₂ to a 1.00 L solution are 0.5 M and 3.0 M, respectively.
The reaction N2O4 ⇌ 2NO2 is given. After the addition of 1.00 mol NO2 to a 1.00 L solution, we are to determine the equilibrium concentrations of N2O4 and NO2.
Initial moles of NO2 = 1.00 mol
Initial concentration of N2O4 = 1.5 M
Using the equation N2O₄ ⇌ 2NO₂, the initial concentration of NO₂ can be calculated as follows:
Initial concentration of NO₂ = (0 × 2)/1.5 = 0 M
From the equation N2O₄ ⇌ 2NO₂, it is known that 1 mole of N2O₄ yields 2 moles of NO₂.
Therefore, the number of moles of N2O₄ that dissociate can be determined:
Initial moles of N2O₄ = (1.5 - x)
Total moles of NO₂ = (2 + x)
2 + x = 3.5 moles
x = 1.5 moles
Hence, 1.5 moles of N2O₄ dissociate to form 3.0 moles of NO₂, leaving 0.5 moles of N2O₄ remaining in equilibrium.
The concentrations of N2O₄ and NO₂, the formula for molar concentration is used:
Concentration = Number of moles / Volume of solution
Concentration of N2O₄ = 0.5 moles / 1 L = 0.5 M (as 0.5 moles of N2O₄ remains)
Concentration of NO₂ = 3.0 moles / 1 L = 3.0 M (as 3.0 moles of NO₂ is formed)
Therefore, the equilibrium concentrations of N2O₄ and NO₂ after the addition of 1.00 mol NO₂ to a 1.00 L solution are 0.5 M and 3.0 M, respectively.
To know more about Equilibrium here: https://brainly.com/question/517289
#SPJ11
NEED ANSWER FAST✴️✴️✴️✴️
What do phytoplankton do for the carbon cycle? Choose all that apply.
A. They take in oxygen for photosynthesis.
B. They take in carbon dioxide for photosynthesis.
C. When they die, the carbon left in them can be sequestered (stored) in the ocean bottom.
D. They are food for many animals, including some whales.
Answers
What is used to determine the number of each atom in an ionic formula
Answers
Answer:
The charge carried by each ion (oxidation state of each atom)
Explanation:
If we have an ionic compound and we want to write its formula, we must first know the magnitude of charge on each ion (shown as oxidation state of the atoms involved) because the magnitude of charge on each ion is eventually crisscrossed and gives the subscript (number of atoms) for each atom in the formula.
For instance, let us write the formula of calcium bromide. Ca has a charge of +2 while Br has a charge of -1. If we exchange the charges and ignore the signs such that the crisscrossed charges form subscripts we can now write; \(CaBr_{2}\).
___is the transfer of heat through the movement of a fluid such as water or air.
Answers
Answer:
Convection
is the movement of heat by a fluid such as water or air. The fluid (liquid or gas) moves from one location to another, transferring heat along with it. This movement of a mass of heated water or air is called a current. Radiation is the transfer of heat by electromagnetic waves.
According to the second law of energy, most energy is wasted in the form of ________
A. sound
B. heat
C. electricity
D. light
Answers
A. sound
B. heat
C. electricity
D. light
Answer is b
how could a carbon atom in coal end up in a penguin
Answers
Answer:
Explanation:
Combustion of coal releases carbon as CO2. Phytoplankton remove carbon dioxide from the atmosphere by photosynthesis. Carbon is passed along the food chain as fish eat the plants. Penguins feed on the fish, taking in the carbon compounds containing the original carbon atom.
The carbon atoms in coal end up in a penguin due to the process of food chain.
What is a penguin?
Penguins are aquatic flightless birds that are found in the Southern Hemisphere.
The food chain leads to the carbon atom into the penguin.
The carbon atoms are release from the coal combustion.
The released atoms go into plants leaves.
The herbivorous eat plants.
Then the carnivorous eats the herbivorous.
The carnivorous can be a fish and fish are eaten by penguins.
Thus, in this way, the carbon atoms in coal end up in a penguin due to the process of food chain.
Learn more about food chain, here:
https://brainly.com/question/16065961
consider the hypothetical atom, with a mass number of 85 and an atomic number of 44, what would be the number of protons (p), neutrons (n), and electrons (e), if the atom was real?
Answers
For the hypothetical atom with a mass number of 85 and an atomic number of 44, a real atom of this isotope (Ruthenium) would have:
Protons (p) = 44
Neutrons (n) = 41
Electrons (e) = 44
The hypothetical atom with a mass number of 85 and an atomic number of 44 represents an isotope of the element Ruthenium (Ru). To determine the number of protons, neutrons, and electrons in a real atom of this isotope, we need to understand the atomic structure.
The atomic number (Z) represents the number of protons in an atom. Since the atomic number is given as 44, the number of protons (p) in the atom is 44.
The mass number (A) represents the total number of protons and neutrons in the nucleus of an atom. In this case, the mass number is given as 85. Therefore, the number of neutrons (n) can be calculated by subtracting the atomic number (protons) from the mass number:
Neutrons (n) = Mass number (A) - Atomic number (Z)
Neutrons (n) = 85 - 44
Neutrons (n) = 41
To determine the number of electrons (e), we assume that the atom is neutral, meaning it has an equal number of protons and electrons. Therefore, the number of electrons is also 44.
For more such question on isotope visit;
https://brainly.com/question/14220416
#SPJ8
Which is the best way to determine if an object is made of pure silver?
Answers
Answer:
The Nitric Acid Test is used to check if silver is pure or plated. To do so, file a small part of the item in a discreet area where it cannot be seen. Apply a few drops of nitric acid. If the area turns into creamy white, the silver is pure or sterling.
Explanation:
What is wrong with the formula CH3CH2CH=CH3
1. The formula has too many H atoms bound to the right-most C atom
2. the formula has too many H atoms bound to the left-most C atom
3. The formula has too many H atoms bound to the second C atom
4. The formula is correct
Answers
1. The formula has too many H atoms bound to the right-most C atom.
Explanation:
Carbon atoms can only have four bonds. = means a double bond so there should only be 2 H atoms as two bonds are already taken up by the last Carbon.
My car has an internal volume of 12,000 L. If I drive my car into the river and it implodes, what will be the volume of the gas when the pressure goes from 1.0 atm to 1.4 atm?
Answers
The volume of gas when the pressure goes from 1.0 atm to 1.4 atm is 8,571.43 L.
When a car is driven into the river, it will implode due to the change in pressure. We are to calculate the volume of gas when the pressure goes from 1.0 atm to 1.4 atm if the internal volume of the car is 12,000 L.In order to solve the problem, we will use the combined gas law equation. The equation is given as follows;P1V1/T1 = P2V2/T2where P1 is the initial pressure, V1 is the initial volume, T1 is the initial temperature, P2 is the final pressure, V2 is the final volume, and T2 is the final temperature.We will assume that the initial temperature and final temperature are constant, and therefore, we can cancel them from the equation. Thus, the equation becomes;P1V1 = P2V2We can rearrange the equation to solve for V2 as follows;V2 = (P1V1)/P2Substituting the given values, we get;V2 = (1.0 atm * 12,000 L)/1.4 atmV2 = 8,571.43 L.
For more questions on pressure
https://brainly.com/question/24719118
#SPJ8
Which of the following accurately describes metallic bonding?
A. electrons sea of delocalized
B. electrons are shared between atoms
C. electrons are transferred between atoms
D. electrons are released as beta particles
The answer is not C that's all I know. Please answer with explanation if you know.
Answers
Answer:
A
Explanation:
write both the complete ionic equation and Net ionic equation for aluminum sulfate and sodium carbonate ( Al2(SO4)3 + Na2CO3 )
Answers
2Al3+(aq) + 3SO42-(aq) + 2Na+(aq) + CO32-(aq) → Al2(CO3)3(s) + 3SO42-(aq) + 2Na+(aq)
The net ionic equation is obtained by eliminating the spectator ions (ions that appear on both sides of the equation and do not participate in the reaction). The spectator ions in this reaction are Na+ and SO42-. Therefore, the net ionic equation is:
2Al3+(aq) + 3CO32-(aq) → Al2(CO3)3(s)
Which statement is true of a reversible reaction at equilibrium?
А.
The concentration of reactants is less than the concentration of products.
B.
The concentration of reactants and the concentration of products are equal.
с.
The concentration of reactants is greater than the concentration of products.
D.
The concentration of reactants and the concentration of products are constant.
Ε.
The concentration of reactants is decreasing and the concentration of products is increasing,
Answers
Answer:
D.
The concentration of reactants and the concentration of products are constant.
Explanation:
pls mark as brainliest
When a chemical system is at equilibrium, the concentrations of the reactants are equal to:___________.
1. the concentrations of the products.
2. the concentrations of the reactants and products have reached constant values.
3. the forward and reverse reactions have stopped. the reaction quotient, , has reached a maximum.
4. the reaction quotient, , has reached a minimum.
Answers
Answer:
catalyst
Explanation:
how to make sweetened condensed milk from evaporated milk?
Answers
Answer:
Just combine one 12-oz can of evaporated milk and 1-1/2 cups granulated sugar in a sauce pan. Bring the mixture to a boil over medium heat, stirring constantly. Continue cooking, until the sugar dissolves, and the milk thickens slightly. Allow your sweetened condensed milk to cool.
Explanation:
hope this helps
Answer:
Mix one 12-oz can of evaporated milk and 1-1/2 cups granulated sugar. :)
How does the amount of oxygen in inhaled air compare to the amount of oxygen in exhaled air? a. there is 21% oxygen in inhaled air, 78% in exhaled air. b. there is 0% oxygen in exhaled air. c. there is 78% oxygen in inhaled air, 15% in exhaled air. d. there is 21% oxygen in inhaled air, 15% in exhaled air.
Answers
There is 21% oxygen in inhaled air and 15% oxygen in exhaled air.
What is oxygen?The chemical element with the atomic number 8 and symbol O is called oxygen.It belongs to the periodic table's chalcogen group, is a very reactive nonmetal, and an oxidizing agent that easily produces oxides with most elements as well as other compounds.After hydrogen and helium, oxygen is the third most plentiful element in the universe and the most abundant element on Earth.Two atoms of the elements combine to generate dioxygen, a colorless and odorless diatomic gas, at ordinary temperature and pressure.The Earth's atmosphere is currently made up of 20.95% diatomic oxygen gas, though this has fluctuated significantly over a very long time. The Earth's crust is almost 50% oxygen in the form of oxides.To learn more about Oxygen with the given link
https://brainly.com/question/2272415
#SPJ4