Never heard of this!!
Please help me I had a really hard day my brain is breaking apart


Answers
Answer:
See explanation
Explanation:
a) u= 60 m/s, v= 45 m/s, t = 7 secs
From;
v = u + at
a = v - u/t
a= 45 - 60/7
a = -2.14 m/s^2
b)
u = 200 m/s
v= 235 m/s
t = 30 secs
v = u + at
a = v-u/t
a = 235 - 200/30
a= 1.17 m/s^2
c)
i) Acceleration = 3 - 1/2-0 = 2/2 = 1 m/s^2
ii) The slope between 2 and 5 is zero because velocity was not changing with time.
iii) Within the time between 2 and 5 the speed remained at 3 m/s
iv) the slope between 5 and 8 is 2 - 3/8 - 5 = -1/3 = -0.33m/s^2
v) The negative slope means that velocity is decreasing with time(deceleration).
Related Questions
Electric current flow is the result of the movement of ______. Select all that apply. a) anions b) cations c) electrons d) molecules.
Answers
Electric current flow is the result of the movement of b) cations and c) electrons.
Cations are positively charged ions that move towards the negative electrode, while electrons are negatively charged particles that move towards the positive electrode.
Anions, negatively charged ions, do not contribute to electric current flow as they move towards the positive electrode, opposite to the direction of electron flow.
Molecules, which are made up of atoms and can be charged or uncharged, do not move as a whole in electric circuits but their constituent particles, such as electrons and cations, may contribute to current flow. Therefore, options a) and d) are incorrect.
For more questions like Current click the link below:
https://brainly.com/question/2264542
#SPJ11
what mass of aluminum has a total nuclear charge of 1.1 c? aluminum has atomic number 13. suppose the aluminum is all of the isotope with 14 neutrons.
Answers
The total nuclear charge of an atom is equal to the number of protons in the nucleus, which is also known as the atomic number. The atomic number of aluminum is 13, which means that aluminum has 13 protons in its nucleus. 1.6858 x 10⁻¹⁸ kg of aluminum has a total nuclear charge of 1.1 C.
The isotope of aluminum with 14 neutrons is known as aluminum-27, and its atomic mass is equal to 13 protons + 14 neutrons = 27 atomic mass units.
We can calculate the mass of aluminum with a total nuclear charge of 1.1 C by using the following formula:
mass = (total nuclear charge) / (elementary charge) x (atomic mass)
where the elementary charge is 1.602176634 x 10⁻¹⁹ C.
Plugging in the values, we get:
mass = (1.1 C) / (1.602176634 x 10⁻¹⁹ C) x (27 atomic mass units)
mass = 1.6858 x 10⁻¹⁸ kg
Therefore, 1.6858 x 10⁻¹⁸ kg of aluminum has a total nuclear charge of 1.1 C.
The nuclear charge, also known as the atomic number, is the number of protons in the nucleus of an atom. The protons are positively charged particles, and the number of protons in an atom determines the atomic number and the identity of an element. The nuclear charge of an atom determines its chemical behavior, as elements with the same number of protons are chemically similar.
Learn more about Nuclear charge:
brainly.com/question/13664060
#SPJ4
A solution of sodium hydroxide was titrated against a solution of sulfuric acid. How many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers
Answer:
2 mole of Sodium hydroxide reacts with 1 mole of Sulfuric acid
Explanation:
Write down the equation in the beginning with reactants and products:
NaOH + H₂SO₄ → Na₂SO₄ + H₂0
Now try to balance it. Try with Na first:
2NaOH + H₂SO₄ → Na₂SO₄ + H₂0
Na atoms are balanced. There are 6 Oxygen atoms on the right and 5 on the left. Balance by increasing the H₂O moles:
2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂0
Check if H atoms are also balanced. They are. That means our final reaction is:
2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂0
2 Moles of NaOH reacts with 1 mole of H₂SO₄
The moles of sodium hydroxide required to neutralize 1 mole of sulfuric acid have been 2 mol.
The balanced chemical equation for the titration of sodium hydroxide against sulfuric acid has been:
\(\rm 2\;NaOH\;+\;H_2SO_4\;\rightarrow\;Na_2SO_4\;+\;2\;H_2O\)
From the balanced chemical equation, for the titration of 1 mole of sulfuric acid, 2 moles of sodium hydroxide has been required.
The moles of sodium hydroxide required for the titration of sulfuric acid has been:
\(\rm 1\;mol\;H_2SO_4=2\;mol\;NaOH\)
Thus, the neutralization of 1 mole of sulfuric acid has been required 2 mol of sodium hydroxide.
For more information about titration, refer to the link:
https://brainly.com/question/25485091
Question is in photo! Will give BRAINLIEST to whoever answers correctly first!

Answers
Answer:
A new element
Explanation:
As you add mopre and more protons and electrons and neutrons it keeps on crating new.
A wave's frequency is 5.00x10^14 s-1.
What is the wavelength?
Answers
The wavelength of the wave having a frequency of 5.00×10¹⁴ s⁻¹ is 6×10⁻⁷ m
How do I determine the wavelength?We know that the velocity is related to wavelength and frequency according to the following formula:
Velocity (v) = wavelength (λ) × frequency (f)
v = λf
Thus, with the above formula, we can determine the wavelength. Details below:
Frequency (f) = 5.00×10¹⁴ s⁻¹Speed of wave (v) of = 3×10⁸ m/sWavelength (λ) = ?Velocity (v) = wavelength (λ) × frequency (f)
3×10⁸ = wavelength × 5.00×10¹⁴
Divide both sides by 5.00×10¹⁴
Wavelength = 3×10⁸ / 5.00×10¹⁴
Wavelength = 6×10⁻⁷ m
Therforee, the wavelength is 6×10⁻⁷ m
Learn more about wavelength:
https://brainly.com/question/13659036
#SPJ1
If a chemist wants to make 50g of HClO3, what is the minimum number of grams of ClO2 that she must use?
the reaction is 6ClO2+3H2O=5HClO3+HCl
Answers
Taking into account the reaction stoichiometry, the minimum number of grams of ClO₂ that a chemistry must use to make 50 g of HClO₃ is 47.92 grams.
Reaction stoichiometryIn first place, the balanced reaction is:
6 ClO₂ + 3 H₂O → 5 HClO₃ + HCl
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
ClO₂: 6 molesH₂O: 3 molesHClO₃: 5 molesHCl: 1 moleThe molar mass of the compounds is:
ClO₂: 67.45 g/moleH₂O: 18 g/moleHClO₃: 84.45 g/moleHCl: 36.45 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
ClO₂: 6 moles ×67.45 g/mole= 404.7 gramsH₂O: 3 moles ×18 g/mole= 54 gramsHClO₃: 5 moles ×84.45 g/mole= 422.25 gramsHCl: 1 mole ×36.45 g/mole= 36.45 gramsMass of ClO₂ requiredThe following rule of three can be applied: If by reaction stoichiometry 422.25 grams of HClO₃ are formed from 404.7 grams of ClO₂, 50 grams of HClO₃ are formed from how much mass of ClO₂?
mass of ClO₂= (50 grams of HClO₃× 404.7 grams of ClO₂)÷ 422.25 grams of HClO₃
mass of ClO₂= 47.92 grams
Finally, 47.92 grams of ClO₂ is required.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
brainly.com/question/24653699
#SPJ1
Place the following in order of increasing X-A-X bond angle, where A represents the central atom and X represents the outer atoms in each molecule.
HCN H2O H3o+
H2O < H3O+ < HCN
H3O+ < H2O < HCN
H3O+ < HCN < H2O
HCN < H3O+ < H2O
H2O < HCN < H3O+
Answers
The correct order of increasing X-A-X bond angle is: H3O+ < HCN < H2O.
To determine the order of increasing X-A-X bond angle, we need to consider the molecular geometries of the given molecules: HCN, H2O, and H3O+.
1. HCN: In HCN, the central atom is carbon (C) bonded to a hydrogen atom (H) and a nitrogen atom (N). The molecule has a linear geometry, which means the X-A-X bond angle is 180°.
2. H2O: In H2O, the central atom is oxygen (O) bonded to two hydrogen atoms (H). The molecule has a bent or V-shaped geometry due to the presence of two lone pairs of electrons on oxygen. This lone pair repulsion causes a decrease in the bond angle. The X-A-X bond angle in H2O is approximately 104.5°.
3. H3O+: In H3O+, the central atom is also oxygen (O) bonded to three hydrogen atoms (H). The molecule has a trigonal pyramidal geometry, with the hydrogen atoms positioned around the central oxygen atom. The presence of the positively charged oxygen (H3O+) further distorts the geometry, resulting in a smaller bond angle compared to H2O. The X-A-X bond angle in H3O+ is less than 104.5°.
Therefore, the correct order of increasing X-A-X bond angle is: H3O+ < HCN < H2O.
To know more about molecular geometries refer here:
https://brainly.com/question/31993718#
#SPJ11
The ideal Relative Humidity \( (\mathrm{RH}) \) is (___) \% in the building. 65 50 35 30
Answers
The ideal Relative Humidity (RH) is 50% in the building. Relative Humidity is the amount of water vapor in the air compared to the amount of moisture that the air can hold at a particular temperature.
The ideal RH level for human comfort and wellness is between 30% and 60%.
A level below or above this range can cause several problems for humans as well as the environment. RH levels below 30% can cause issues such as dry skin, eyes, and mucous membranes, whereas levels above 60% can cause allergies, mildew, and mold.RH is also critical in industrial production facilities and laboratories, where there is a need to maintain strict control over environmental conditions.
Maintaining a specific humidity level is essential for the correct operation of certain types of machinery, equipment, and tools.RH levels also have an impact on the energy efficiency of buildings, as higher humidity levels can increase the amount of energy required to cool or heat the building.
Therefore, it is essential to maintain optimal RH levels to ensure human health and comfort and the efficient functioning of equipment and machinery. In conclusion, the ideal Relative Humidity (RH) in the building is 50%.
Learn more about Humidity here,
https://brainly.com/question/30765788
#SPJ11
How many atoms of O are present in 2CaCO3?
Answers
Answer:
there are 6 atoms of oxygen in this compound
Explanation:
2CaCO₃ Oxygen= 2(which multiplies every element) × 3(which is the no of atoms just oxygen alone.) that is equal to six 6
The freezing point of pure water is 0.0 °C. In the previous step, you calculated that the freezing point changes by 1.09 °C. What is the new freezing point of the solution? FP = [?] °C Hint: Remember significant figures are based on place value when adding or subtracting.
Answers
The new freezing point of the solution is 1.09 °C.
Based on the given information, the freezing point of pure water is 0.0 °C, and the freezing point changes by 1.09 °C. To find the new freezing point of the solution, we need to add the change in freezing point to the freezing point of pure water.
0.0 °C + 1.09 °C = 1.09 °C
Therefore, the new freezing point of the solution is 1.09 °C.
When performing calculations with significant figures, it's important to consider the rule for addition and subtraction. According to this rule, the result should be rounded to the least number of decimal places among the values being added or subtracted. In this case, both the freezing point of pure water (0.0 °C) and the change in freezing point (1.09 °C) have one decimal place. Thus, the final answer is also rounded to one decimal place, resulting in a new freezing point of 1.1 °C.
for more such question on freezing visit
https://brainly.com/question/24314907
#SPJ8
HELP PLEASE if u answer ur swag

Answers
Answer:
E = 1.3*10^-18 J
Explanation:
E = hv
E = 6.626*10^-34 Js * 2 *10^15 s-1
E = 13.252*10^-19 J
E = 1.3*10^-18 J
Answer:
2 ×10¹⁵ × 6.626 × 10^-34= 13.25 × 10^-19
= 1.3 × 10^-18
Explanation:
The energy associated with an electromagnetic wave is given by Planck's constant (6.626 x 10-34 Js) times its frequency.
Which set of quantum numbers cannot specify an orbital?.
Answers
Answer: n=2,l=1,ml=1 n=3,l=2,ml=0 n=3,l=3,ml=2 n=4,l=2,ml=0.
Explanation:
hope it helps
all restaurant kitchens are equipped with what kind of systems that release wet chemicals directly over the source of the fire when smoke is detected?
Answers
Every restaurant kitchen has a hood suppression system that, when smoke is detected, releases wet chemicals over the fire's source.
Wet chemical method: what is it?Wet chem is a type of analytical methods that examines materials using traditional techniques like observation. Wet chemistry is the term used to describe the majority of analysis, which takes place in the liquid phase. Because many tests are carried out at lab benches, wet chemistry is sometimes known as bench chemistry.
Wet chemical agents: what are they?Chemical wet The extinguishing agent may consist of water in solutions with potassium dichromate, potassium carbonate, potassium lactate, or a combination of these compounds, but this is not a requirement (which are conductors of electricity). The pH of the liquid agent is normally 9.0.
To know more about Wet chemicals visit :
https://brainly.com/question/28260537
#SPJ4
hydrochloric acid + __________ —> copper chloride + water
Pls say someone answer science iam confused
Answers
Explanation:
HCl + __ => CuCl2 + H2O
The blank should be the metal, which is copper.
(This reaction is not spontaneous but can happen using electrolysis, etc.)
sodium is a mineral element and is one of the principle positive ions. T/F?
Answers
True. Sodium is indeed a mineral element that is one of the principle positive ions, also known as cations, in the human body. It plays a crucial role in various bodily functions such as maintaining proper fluid balance, transmitting nerve impulses, and supporting muscle function.
Sodium can be found in a variety of foods, particularly in salt (sodium chloride), processed foods, and certain types of dairy products. However, it's important to consume sodium in moderation as excessive intake can lead to negative health effects such as high blood pressure and increased risk of cardiovascular disease. True, sodium is a mineral element and is one of the principle positive ions.
Sodium is indeed a mineral element and is one of the principle positive ions, or cations, in our body. It plays a crucial role in maintaining proper fluid balance, nerve function, and muscle function. Sodium is an essential element that our body needs for various physiological processes. As a positive ion, it helps in maintaining the balance of fluids inside and outside our cells. Sodium also plays a critical role in transmitting nerve impulses and aiding muscle contractions.
To know more about element visit :
https://brainly.com/question/31950312
#SPJ11
If ph of a solution is 11.2, Find Conc. of [OH-] ions
Answers
If the pH of a solution is 11.2, then the concentration of [OH-] is 1.585×10³M.
The pH of a solution is a measure of the concentration of hydrogen ions (H+), which in turn is a measure of acidity.
Given : pH of a solution=11.2
we know that pH + pOH = 14
pOH = -log[OH-]
pOH = 14 - pH
= 14 - 11.2
pOH = 2.80
-log[OH-] = 2.80
log[OH-] = -2.80
[OH-] = 10∧-2.80
[OH-] = 1.585 ×10∧-3 M.
Please help ASAP.....please
would you choose to buy a soft drink in a plastic bottle or an aluminium can? Explain your answer.(3 marks)
Answers
Answer:
In a bottle.
Explanation:
I would like soda in a bottle because you don't get soda stuck in the aluminum can like you do a bottle. And it's also way more efficient because bottles have caps that you can screw on later, but aluminum doesn't.
Ayúdenme por favor
Please help me
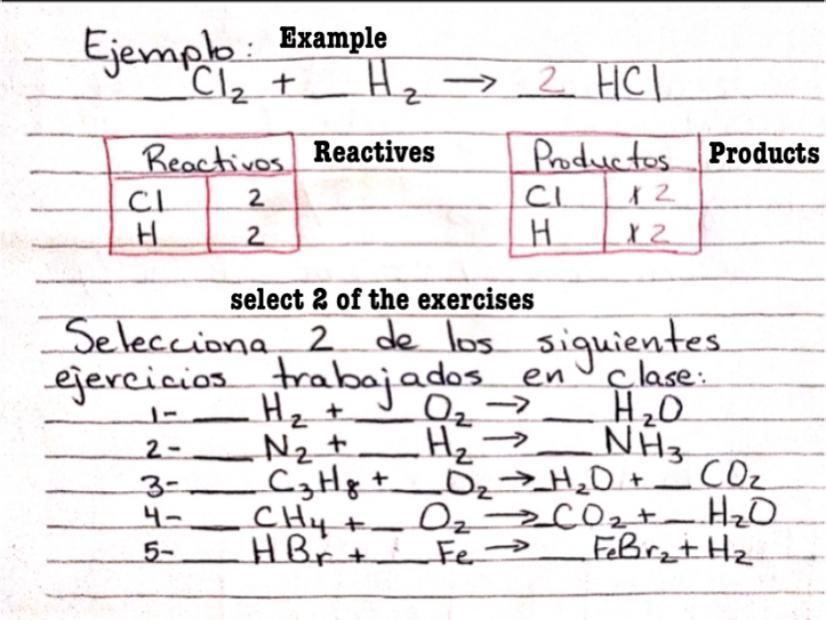
Answers
2H2+O2===>2H2O
N2+3H2==>2NH3
C3H8+5O2==>4H20+3CO2
CH4+2O2===>CO2+2H2O
2HBr +Fe==>FeBr2 +H2
I hope it helped
Does this particle diagram represent an element, compound, or mixture?

Answers
Which of the following combinations could be linked together to form a nucleotide?
A) 1, 2, and 11
B) 3, 7, and 8
C) 5, 9, and 10
D) 11, 12, and 13
E) 12, 14, and 15
Answers
In order to produce a nucleotide, the combinations 11, 12, and 13 may be combined.
The definition and purpose of a nucleotideThe basic unit of DNA and RNA are nucleotides. Genetic material is present in them. Since many biological reactions involving enzymes require coenzymes, nucleotides serve in this capacity. ATP serves as the body's energy storage system.
How does DNA get made up of nucleotides?A phosphodiester bond forms when the 5' phosphate group of one nucleotide and the 3'-OH group of another nucleotide come together during the incorporation of nucleotides into DNA (see below). Phosphate-sugar-phosphate-sugar-phosphate forms the "backbone" of each DNA strand in this way.
To know more about Nucleotides visit:
https://brainly.com/question/28250277
#SPJ4
calculate the average kinetic and potential energies of an electron in the ground state of a hydrogen atom
Answers
9.27 × 10⁻¹⁹ J and -2.18 × 10⁻¹⁸ J is the average kinetic and potential energies of an electron in the ground state of a hydrogen atom.
In order to calculate the average kinetic and potential energies of an electron in the ground state of a hydrogen atom, we can use the following equations:
Kinetic energy: K = (1/2)mv²
where m is the mass of the electron and v is its velocity.
Potential energy: U = -E
where E is the energy of the electron, which can be found using the equation:
E = -13.6/n²
where n is the principal quantum number for the electron in the ground state of the hydrogen atom.
So, we need to find the mass and velocity of the electron. The mass of an electron is approximately 9.11 × 10⁻³¹ kg, and the velocity can be found using the equation:
v = (Zke²/r)m
where Z is the atomic number of hydrogen (1), k is Coulomb's constant, e is the elementary charge, and r is the Bohr radius, which is equal to 0.529 Å.
Substituting these values, we get:
v = (1 × 8.99 × 10⁹ N·m²/C² × 1.60 × 10⁻¹⁹ C² / (0.529 × 10⁻¹⁰ m)) / (9.11 × 10⁻³¹ kg) = 2.18 × 10⁶ m/s
Now, we can use these values to calculate the average kinetic energy:
K = (1/2)mv² = (1/2)(9.11 × 10⁻³¹ kg)(2.18 × 10⁶ m/s)² = 9.27 × 10⁻¹⁹ J
And the potential energy:
U = -E = -(-13.6 eV) = 13.6 eV × 1.60 × 10⁻¹⁹ J/eV = -2.18 × 10⁻¹⁸ J
Therefore, the average kinetic energy is 9.27 × 10⁻¹⁹ J and the average potential energy is -2.18 × 10⁻¹⁸ J.
To know more about Electron refer here:
https://brainly.com/question/28337734
#SPJ11
In a certain reaction, 27.3 g of iron reacts with 45.8 g of oxygen. What is the limiting reactant?
Answers
The limiting reactant for the reaction between 27.3 g of iron and 45.8 g of oxygen is iron
Balanced equationWe'll begin by writing the balanced equation for the reaction between iron and oxygen. This is given below:
4Fe + 3O₂ --> 2Fe₂O₃
Molar mass of Fe = 56 g/mol
Mass of Fe from the balanced equation = 4 × 56 = 224 g
Molar mass of O₂ = 16 × 2 = 32 g/mol
Mass of O₂ from the balanced equation = 3 × 32 = 96 g
SUMMARY
From the balanced equation above,
224 g of Fe reacted with 96 g of O₂
How to determine the limiting reactantThe limiting reactant can be obtain as illustrated below:
From the balanced equation above,
224 g of Fe reacted with 96 g of O₂
Therefore,
27.3 g of Fe will react with = (27.3 × 96) / 224 = 11.7 g of O₂
From the calculation above, we can see that only 11.7 g of O₂ out of 45.8 g given is required to react completely with 27.3 g of Fe.
Thus, Fe is the limiting reactant.
Learn more about stoichiometry:
https://brainly.com/question/11587316
#SPJ1
what is the ph of 1.00 l of rainwater that has dissolved 4.18 mg of no2 ? assume that all of the no2 has reacted with water to give nitric acid.
Answers
The pH of the solution is 1.954. The pH scale ranges from 0 to 14, with 7 being neutral.
The pH of a solution is defined as the negative logarithm (base 10) of the concentration of hydrogen ions (H+) in the solution. Since HNO3 is a strong acid, it is almost completely dissociated in water, so the concentration of H+ ions in the solution is equal to the concentration of HNO3.
We first need to determine the concentration of nitric acid (HNO3) that has formed from the reaction of NO2 with water.
The molar mass of NO2 is 46.006 g/mol, so the number of moles of NO2 dissolved in 1.00 L of water is
4.18 mg / 46.006 g/mol = 0.0910 mol.
Since the reaction between NO2 and water is:
NO2 (aq) + H2O(l) → HNO3 (aq) + NO(g)
The number of moles of HNO3 formed is equal to the number of moles of NO2 present, which is 0.0910 mol.
So, the concentration of HNO3 is 0.0910 mol / 1.00 L = 0.0910 M.
pH = -log(0.0910) =1.954
Learn more about pH, in here: https://brainly.com/question/15289741
#SPJ4
Pls answer I am confused PLEASE HELP

Answers
Answer:
Glucose is the carbohydrate produced by photosynthesis. Energy-rich glucose is delivered through your blood to each of your cells. ATP is the usable form of energy for your cells.
Explanation:
Calculate the volume of an
object with the following
dimensions:
5.0 cm x 16 cm x 12 cm
Answers
Answer:
960cm
Explanation:
5.0cm x 16cm =80cm
80cm x 12cm=960
Hope this helps : )
A 5.0-gram sample of octane (C₂H₁g) is burned in a calorimeter containing 1200 grams of
water. The water temperature rises from 25°C to 41.5°C. Calculate the AH for this reaction in
kilocalories
Answers
The enthalpy change, ΔH, for the reaction, given that 5 grams of octane, C₈H₁₈ is burned in the calorimeter containing 1200 g of water is 450 Kcal/mol
How do i determine the change in enthalpy?First, we shall determine the mole of 5 grams of octane, C₈H₁₈. Details below:
Mass of C₈H₁₈ = 5 grams Molar mass of C₈H₁₈ = 114 g/mol Mole of C₈H₁₈ =?Mole = mass / molar mass
Mole of C₈H₁₈ = 5 / 114
Mole of C₈H₁₈ = 0.044 mole
Next, we shall obtain the heat absorbed by the water. Details below:
Mass of water (M) = 1200 gInitial temperature of water (T₁) = 25 °CFinal temperature of water (T₂) = 41.5 °CChange in temperature of water (ΔT) = 41.5 - 25 = 16.5 °CSpecific heat capacity of water (C) = 1 Cal/gºC Heat (Q) =?Q = MCΔT
Q = 1200 × 1 × 16.5
Q = 19800 cal
Finally, we shall determine the enthalpy change, ΔH, for the reaction. Details below:
Mole of C₈H₁₈ (n) = 0.044 moleHeat involved (Q) = 19800 cal = 19800 / 1000 = 19.8 KcalEnthalpy change (ΔH) =?ΔH = Q / n
ΔH = 19.8 / 0.044
ΔH = 450 Kcal/mol
Thus, the enthalpy change, ΔH for the reaction is 450 Kcal/mol
Learn more about enthalpy change:
https://brainly.com/question/24170335
#SPJ1
How many molecules of ammonia would there be in 40.0 grams of ammonia?
Answers
1.42x10^24 molecules
40.0 grams NH3 (6.02x10^23/17.0 grams NH3)= 1.42x10^24 molecules
When sulfuric acid is combined with sugar, gas is released and a tall black column forms. This is an example of a..?
a. physical property
b. chemical property
c. physical change
d. chemical change
Answers
Answer: d)
Explanation: Sulfuric acid on reaction with sugar leads to the formation of a lot of heat indicating it to be an exothermic reaction, a large quantity of steam , a black carbon containing tube like structure pushing itself out of the beaker.It is demonstrated as:
C12H22O11 (sugar) + H2SO4 (sulfuric acid) → 12 C (carbon) + 11 H2O (water) + mixture water and acid
The process is actually the dehydration of sugar. It can be demonstrated by simply adding table sugar to a beaker and adding sulfuric acid to it. The process is followed by the release of heat, oxide fumes, and a black tube structure as mentioned. The white sugar on addition of this sulfuric acid get dehydrated and pushes itself outwards by forming a black carbon containing tube like structure.
Since the process is irreversible is nature therefore is a chemical change.
Further reference on Sulfuric acid can be taken from:
brainly.com/question/1107054
About how old is the Sun?
1.2 billion years
4.6 billion years
6.4 billion years
8.2 billion years
Answers
Answer:
B : 4.6 billion years
Credits go to the person above me.
;)
Explanation:
EDGE 2021
The sun is about 4.6 billion years old. Therefore, option (B) is correct.
What is the sun made of?The Sun is made primarily of the elements hydrogen and helium. They account for 74.9% and 23.8% mass of the Sun in the photosphere. All heavier elements, called metals account for less than 2% of the mass, with oxygen (<1%), carbon (0.3%), neon (0.2%), and iron (0.2%) being the most abundant.
The hydrogen and helium in the Sun would have been produced by Big Bang nucleosynthesis and the heavier elements were produced by former generations of stars before the Sun was formed.
The amount of helium and its location within the Sun has gradually changed over the past 4.6 billion years. The proportion of helium has raised from about 24% to 60% due to fusion, and some of the He and heavy elements have settled from the photosphere toward the center due to gravity.
Therefore, the age of the sun is about 4.6 billion years.
Learn more about Sun, here:
https://brainly.com/question/2526507
#SPJ6
How many electrons can occupy a filled 3p sublevel?.
Answers
A maximum of 6 electrons can occupy a filled 3p sublevel.
In quantum mechanics, each electron shell is further divided into subshells, which are designated using the letters s, p, d, and f. The p sublevel has three orbitals, and each orbital can hold up to two electrons. As a result, the p sublevel can accommodate a maximum of six electrons. In a neutral atom, the number of electrons is equal to the atomic number.
Therefore, the number of electrons that can occupy a filled 3p sublevel is determined by the number of electrons present in the atom. If an atom has 19 electrons, for example, the first 18 electrons will fill the 1s, 2s, 2p, 3s, and 3p sublevels, and the remaining electron will be present in the 4s sublevel.
Learn more about electron shell here:
https://brainly.com/question/29584809
#SPJ11