Answers
Answer:
d. Light energy to chemical energy
Explanation:
Why is this true?This is because photosynthesis converts light energy into chemical energy in the form of sugars or other carbon compounds.
The other options are not correct because they do not match the actual steps of photosynthesis. Chemical energy to light energy is the reverse of what happens in photosynthesis. Light energy to electrical energy and electrical energy to light energy are not involved in photosynthesis at all.
During photosynthesis, plants convert light energy into chemical energy. Therefore, the correct option is d) Light energy to chemical energy.
In photosynthesis, plants use pigments, such as chlorophyll, to absorb light energy from the sun. This light energy is then used to power a series of chemical reactions in the chloroplasts of plant cells. These reactions involve the conversion of carbon dioxide and water into glucose (a sugar) and oxygen.
The process can be summarized as follows:
1. Light energy from the sun is absorbed by chlorophyll molecules in the chloroplasts of plant cells.
2. This absorbed light energy is used to split water molecules into hydrogen ions (H+) and oxygen atoms.
3. The hydrogen ions are then combined with carbon dioxide (CO2) from the air to produce glucose (C6H12O6).
4. Oxygen gas (O2) is released as a byproduct of photosynthesis.
Overall, photosynthesis is an essential process for plants as it enables them to convert light energy into chemical energy in the form of glucose. This chemical energy can then be used by the plant for growth, reproduction, and other metabolic processes.
Related Questions
Where does the oil in cars come from?
Select 2 that apply
-Phytoplankton convert the sugar created through photosynthesis into oil.
-Coral Reefs clean the water by removing any carbon waste, they then convert this into oil.
-Every time there is an earthquake, oil is being produced and new oil deposits are created.
-The phytoplankton that is dead become liquefied over time due to pressure, and oil deposits
are formed.
-Fish produce oil as a waste product that eventually forms oil deposits.
Answers
- The phytoplankton that is dead become liquefied over time due to pressure, and oil deposits are formed.
- Fish produce oil as a waste product that eventually forms oil deposits.
Enter the balanced complete ionic equation for HCl(aq)+K2CO3(aq)→H2O(l)+CO2(g)+KCl(aq)
Answers
Answer:
2HCl(aq)+K2CO3(aq)→H2O(l)+CO2(g)+2KCl(aq)
Explanation:
HCl(aq)+K2CO3(aq)→H2O(l)+CO2(g)+KCl(aq)
2HCl(aq)+K2CO3(aq)→H2O(l)+CO2(g)+2KCl(aq)
H-1*2=2 H-2
Cl-1*2=2 Cl- 1*2=2
K -2 K-1*2=2
C- 1 C-1
O - 3 O-3
Briefly explain how will you describe which object is moving fast and which one is moving slow?
Answers
Hope that helps it was on googIe
Use this equation for the next question:
2NaOH + H2SO4 ® Na2SO4 + 2H20
If a reaction produces 0.75 moles Na2SO4, how many moles of NaOH were used?
0.75 moles NaOH
2 moles NaOH
.375 moles NaOH
1.5 moles NaOH
Answers
How much energy is required to lower the temperature of 32.45 grams of water by 4.05 oC?
Answers
According to the specific heat capacity, 37752.33 J of energy is required to lower temperature of water.
What is specific heat capacity?Specific heat capacity is defined as the amount of energy required to raise the temperature of one gram of substance by one degree Celsius. It has units of calories or joules per gram per degree Celsius.
It varies with temperature and is different for each state of matter. Water in the liquid form has the highest specific heat capacity among all common substances .Specific heat capacity of a substance is infinite as it undergoes phase transition ,it is highest for gases and can rise if the gas is allowed to expand.
It is given by the formula ,
Q=mcΔT
It is calculated as, Q=32.45×4.2×277=37752.33 J.
Thus, 37752.33 J of energy is required to lower temperature of water.
Learn more about specific heat capacity,here:
https://brainly.com/question/2530523
#SPJ1
68.3 grams of sodium hydroxide reacts with 78.3 grams of magnesium nitrate. ____ grams of magnesium hydroxide will form from this reaction, and ____ (compound) is the limiting reagent.
Blank 1:
Blank 2:
Answers
Answer:
30.8 grams of magnesium hydroxide will form from this reaction, and magnesium nitrate is the limiting reagent.
Explanation:
The reaction that takes place is:
2NaOH + Mg(NO₃)₂ → 2NaNO₃ + Mg(OH)₂Now we convert the given masses of reactants to moles, using their respective molar masses:
68.3 g NaOH ÷ 40 g/mol = 1.71 mol NaOH78.3 g Mg(NO₃)₂ ÷ 148.3 g/mol = 0.528 mol Mg(NO₃)₂0.528 moles of Mg(NO₃)₂ would react completely with (0.528 * 2) 1.056 moles of NaOH. There are more than enough NaOH moles, so NaOH is the reagent in excess and Mg(NO₃)₂ is the limiting reagent.
Now we calculate how many Mg(OH)₂ are produced, using the moles of the limiting reagent:
0.528 mol Mg(NO₃)₂ * \(\frac{1molMg(OH)_2}{1molMg(NO_3)_2}\) = 0.528 mol Mg(OH)₂Finally we convert Mg(OH)₂ moles to grams:
0.528 mol Mg(OH)₂ * 58.32 g/mol = 30.8 gWhat is the main purpose of a leaf
Answers
Explanation:
for respiration(exchange of gases)
Actually, all leaves have the same structure. The main purpose is to carry the photosynthesis out, that provides food that needs to survive. :)
What mass of CO2 is produced when 10.0 g of CH4 is burned in oxygen?
Answers
Taking into account the reaction stoichiometry, 27.5 grams of CO₂ is produced when 10.0 g of CH₄ is burned in oxygen.
Reaction stoichiometryIn first place, the balanced reaction is:
CH₄ + 2 O₂ → CO₂ + 2 H₂O
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
CH₄: 1 moleO₂: 2 molesCO₂: 1 moleH₂O: 2 molesThe molar mass of the compounds is:
CH₄: 16 g/moleO₂: 32 g/moleCO₂: 44 g/moleH₂O: 18 g/moleBy reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
CH₄: 1 mole ×16 g/mole= 16 gramsO₂: 2 moles ×32 g/mole= 64 gramsCO₂: 1 mole ×44 g/mole= 44 gramsH₂O: 2 moles ×18 g/mole= 36 gramsMass of CO₂ formed
It is possible to use a simple rule of three as follows: if by reaction stoichiometry 16 grams of CH₄ form 44 grams of CO₂, 10 grams of CH₄ form how much mass of CO₂?
mass of CO₂= (10 grams of CH₄×44 grams of CO₂)÷16 grams of CH₄
mass of CO₂= 27.5 grams
Finally, 27.5 grams of CO₂ is produced.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
#SPJ1
Vehicle air bags require that a very specific amount of gas is created so that the gas inflates the airbag without causing it to rupture.
Commonly, this is accomplished by decomposing sodium azide (NaN3) to produce sodium metal and nitrogen gas. How much sodium azide is required to produce 80.0 g of nitrogen gas?
Answers
The amount of sodium azide required will be 123.72 grams
Stoichiometric problemFrom the equation of the reaction below:
\(2NaN_3 --- > 2Na + 3N_2\)
The mole ratio of sodium azide to nitrogen gas is 2:3
Mole of 80.0 g nitrogen = 80/28.02 = 2.855 moles
Equivalent mole of sodium azide = 2/3 x 2.855 = 1.903 moles
Mass of 1.903 moles sodium azide = 1.903 x 65 = 123.72 grams
More on stoichiometric problems can be found here: https://brainly.com/question/14465605
#SPJ1
HELP PLS (CHEMISTRY)
How is writing formulas for ionic compounds with transition metals different from the regular metals?
Answers
The first most obvious thing to note is when naming transitional metals, you have to state its charge with roman numerals (except for 1 if I remember correctly). For example, Iron (lll), iron has a charge of 3.
What is the motion of the particles in this kind of wave?
A hand holds the left end of a set of waves. The waves themselves make a larger set of waves in the same direction as that of the smaller waves. A label Wave motion is above the series of waves and an arrow next to the label points right.
The particles will move up and down over large areas.
The particles will move up and down over small areas.
The particles will move side to side over small areas.
The particles will move side to side over large areas.

Answers
Answer:
D
Explanation:
The particles will move side to side over large areas.
Answer:
The particles will move side to side over large areas.
Explanation:
KCIO3 decomposes according to thereaction below:
2KCIO3 → 2KCI + 302
How many moles of O2 form when
2.0 mole of KCIO3 decomposes?
Answers
When 2.0 moles of KCIO3 decompose, 2.0 moles of O2 will form.
The balanced chemical equation shows that 2 moles of KCIO3 decompose to produce 3 moles of O2. Therefore, we can use the stoichiometric ratio from the balanced equation to determine the number of moles of O2 formed when 2.0 moles of KCIO3 decompose.
According to the stoichiometry, 2 moles of KCIO3 produce 3 moles of O2. Therefore, we can set up a proportion:
(2 moles KCIO3 / 2 moles O2) = (2.0 moles KCIO3 / x moles O2),
where x represents the unknown number of moles of O2 formed.
Simplifying the equation:
(2 moles KCIO3 / 2 moles O2) = (2.0 moles KCIO3 / x moles O2),
1 = (2.0 moles KCIO3 / x moles O2),
Cross-multiplying:
x moles O2 = (2.0 moles KCIO3 / 1),
x moles O2 = 2.0 moles KCIO3.
Therefore, when 2.0 moles of KCIO3 decompose, 2.0 moles of O2 will form.
It is important to note that this calculation assumes complete and ideal conditions, where the reaction proceeds with 100% efficiency. In reality, the actual yield of O2 may be lower due to various factors such as side reactions or incomplete decomposition. To determine the actual yield, additional information or experimental data would be required.
For more such questions on decompose visit:
https://brainly.com/question/14608831
#SPJ8
The boiling point at 1.00 atm of an aqueous solution of CaCl2 is 105.3 °C. What is the concentration of CaCl2
in the solution? Kb (H2O) = 0.512 °C/m. Assume an ideal van't Hoff factor for CaCl2.
Answers
d.2.93m.CaCl2 is present in the solution with a 2.93m concentration.
The boiling point of a solution is directly related to its concentration. The boiling point elevation of a solution, ΔTb, is equal to the product of the van't Hoff factor (i) and the molality of the solution (m).The quantity of moles of solute per kilogramme of solvent is known as molality.
Therefore, we can solve for the molality of the solution using the following equation:
ΔTb
\(= i *m\\105.3\°C= i * m\\\)
\(m =\frac{ 105.3 \°C }{i}\)
Assuming an ideal van't Hoff factor for CaCl2 (i = 2), the molality of the solution is:
\(m =\frac{ 105.3 \°C }{ 2}\\m = 52.65 m = 52.65 mol/kg\)
The concentration of CaCl2 in the solution is then:
\(C = m * Kb\\C = 52.65 mol/kg * 0.512 \°C/m\\C = 2.93 mol/kg\)
Therefore,The concentration of CaCl2 in the solution is 2.93m.
learn more about boiling point refer:brainly.com/question/24168079
#SPJ1
complete question:The boiling point at 1.00 atm of an aqueous solution of CaCl2 is 105.3 °C. What is the concentration of CaCl2 in the solution? Kb (H2O) = 0.512 °C/m. Assume an ideal van't Hoff factor for CaCl2.
a.3.45m
b.4.40m
c.8.79m
d.2.93m
Which daughter element is produced from the alpha decay of 213 over 85 At ?
A. 213 over 86 Rn
B.217 over 87 Fr
C. 213 over 84 Po
D. 209 over 83 Bi

Answers
Answer:
209
83 Bi
Explanation:
213 213 - 4 4
85 At = 85 - 2 Y + 2 He


Select the correct answer.
What is the percentage of lithium in lithium carbonate (Li₂CO3)?
O A.
OB. 16.25%
O C.
O D.
9.39%
18.78%
21.65%
Answers
The percent by mass of the lithium in the compound is 18.78%. Option C
What is the percentage?We know that the percentage has to do with the amount of the element that can be found in the compound. We can be able to obtain this when we find the molar mass of the compound and then obtain the mass of the element in the compound.
Hence;
Molar mass of the compound = [2(7) + 12 + 3(16)]
= 14 + 12 + 48
= 74 g/mol
The we have the mass of the lithium in the compound as 14
Thus we then have;
Percent of lithium = 14/74 * 100/1
= 18.78%
Learn more about percent by mass:https://brainly.com/question/5394922
#SPJ1
The coeffi cient of absorption (COA) for a clay brick is the ratio of the amount of cold water to the amount of boiling water that the brick will absorb. The output below from JMP shows a regression analysis predicting COA from the pore volume (in cm3/g) of 7 bricks. Some portions of the output have been omitted. What is the value of beta with hat on top subscript 0, the estimated intercept. Round your answer to four decimal places.
Answers
Can anyone just give me like a summary of the C4 topic, I have a test tmrw and have no idea what I’m doing
Answers
A summary of the C4 plants is given below:
What are C4 Plants?The Hatch-Slack pathway, also known as C4 carbon fixation, is one of three recognized photosynthetic carbon fixation pathways in plants.
The names are a result of Marshall Davidson Hatch and Charles Roger Slack's discovery in the 1960s that some plants, when given 14CO2, integrate the 14C label into four-carbon molecules first.
Corn, sorghum, sugarcane, millet, and switchgrass are examples of C4 plants. C3 plants are often more prolific and efficient at photosynthesizing in cooler conditions because C4 morphological and biochemical adaptations demand more energy and resources from the plant.
Read more about C4 plants here:
https://brainly.com/question/1615974
#SPJ1
what do you understand by physical change ? give example
Answers
Answer:
Physical change are the temporary reversible change in which no new substance is formed.
Example: boiling of milk, melting of wax, cooling water,etc.
The single bond is
1 Shorter than the double bond
2 Stronger than the double bonds
3 Longer than the double bond
4 Shorter than the triple bond
Answers
Explanation:
The greater the number multiplicity of the bond, the shorter the bond becomes.
Therefore the single bond is longer than the double bond. (3)
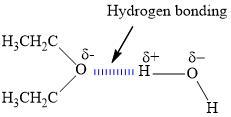
2. How many hydrogen bonds can form between a single ether molecule and water molecules? Draw the structures to explain.
Answers
Ether can only form one hydrogen bond per molecule.
What is hydrogen bonding?We know that the hydrogen bond is the kind of bond that occurs when the dipole of water interacts with the dipole that is on another molecule. We can see this in a lot of hydrides of electronegative elements.
We can see that ether has only one electronegative atom and that is oxygen from the image that is shown in the answer. This oxygen atom can interact with the positive end of the dipole in only one water molecule at a time.
Learn more about hydrogen bonding:https://brainly.com/question/15099999
#SPJ1
Why do we need to identify matter?
Answers
Answer:
It's important for scientists to know the properties of matter because all things are made up of matter. Each type of matter has different physical characteristics and scientists need to know and understand these characteristics to make calculations. ... The main phases of matter are solid, liquid, and gas
Explanation:
^^
Uneven land surfaces created by limestone mining can cause transportation problems in the location. Which of these is the best plan to solve this problem?
water the mining area regularly
reduce the use of heavy machinery in the mining area
choose other uninhabited locations for limestone mining
make reclamation mandatory after limestone has been removed
Answers
Answer:
N. #3
Explanation:
this one just makes the most sense. If you mess with line stone to much or you are using to much heat around lime stone it will go boom. So the correct answer is #3.
In which type of reaction will an acid and a base react with each other?
combustion
neutralization
oxidation-reduction
decomposition
Answers
CHEM FINAL TOMORROW, NEED IMMEDIATE HELP!!! There are a few topics I don't understand, if someone could give a short explanation of how to do these kinds of problems, it would help so much. I'll be posting a few more of these on my page, so feel free to check those out if you would like. Thanks!

Answers
The mass of \(O_2\) needed to produce 8.65 x \(10^{23\) atoms of silver is 22.96 grams.
Stoichiometric calculationIn the given balanced equation, it is stated that 2 moles of \(Ag_2O\) produce 4 moles of Ag and 1 mole of \(O_2\).
First, let's calculate the number of moles of Ag:
8.65 x \(10^{23\) atoms of Ag / (6.022 x \(10^{23\) atoms/mol) = 1.435 moles of Ag
Since 2 moles of \(Ag_2O\) produce 1 mole of \(O_2\), the number of moles of \(O_2\) needed is half of the moles of \(Ag_2O\).
Number of moles of \(O_2\)= 1.435 moles of \(Ag_2O\) / 2 = 0.7175 moles
Now, we'll use the molar mass of \(O_2\) to find the mass:
Molar mass = 32.00 g/mol
Mass of \(O_2\) = number of moles × molar mass
= 0.7175 moles × 32.00 g/mol = 22.96 g
Therefore, the mass of \(O_2\) needed to produce 8.65 x \(10^{23\) atoms of silver is 22.96 grams.
More on stoichiometric calculations can be found here: https://brainly.com/question/27287858
#SPJ1
"Dark Gray had the most consistent temperature at 88°F" Which BEST explains why this a weak evidence statement? "Dark Gray had the most consistent temperature at 88°F" Which BEST explains why this a weak evidence statement? The evidence statement is based on bad/incorrect data. The evidence statement is untrue. The evidence statement is extra information that doesn't help answer the question. The evidence statement contains reasoning. The evidence statement contains a claim.
Answers
Answer:
the evidence statement contains reasoning.
Explanation:
the reason that this piece of information is reasoning rather than evidence is because it doesn't tell us the temperatures of the other substances (if any.) if we look at this substance and it has the most constant temperature compared to the others, how will we know if it is the most constant if we don't know the other substances' data?
this is reasoning because the evidence should tell us more about the substance and the others.
Question 4 of 30
Scientific research shows that Earth's climate is changing due to human
activities. How can scientific research on climate change help society?
A. It can help us stop storms before they occur.
B. It can help us find a new way to make more water.
C. It can help us track how quickly elements of the climate are
changing
D. It can help us reverse the effects of climate change.
th
Answers
C. It can help us track how quickly element of the climate are changing
1. A student mixes two clear, colorless liquids. A magenta colored liquid is formed. Which type of change took place?
A)Nuclear change
B)Chemical change
C)Physical change
D) No change occurred
Answers
Answer:
chemical change because a different solid substance is formed.
Answer:
B) Chemical Change
Explanation:
Combining the two clear colorless liquids is a chemical change because a different solid substance is formed. A precipitate is an insoluble solid that forms when two solutions are combined and react chemically. Insoluble means that the solid will not dissolve.
samples of the gases carbon dioxide =44 and hydrogen =2 ar rhe same temperature,compare the speed of the molecules in these two gases
Answers
Answer:
The speed of molecules in a gas is directly proportional to the square root of the temperature and inversely proportional to the square root of the molar mass.
Since both gases are at the same temperature, we only need to compare their molar masses.
The molar mass of carbon dioxide (CO2) is 44 g/mol and the molar mass of hydrogen (H2) is 2 g/mol.
Therefore, the square root of the molar mass of hydrogen is smaller than the square root of the molar mass of carbon dioxide.
This means that the speed of hydrogen molecules is greater than the speed of carbon dioxide molecules at the same temperature.
In your own words, describe Hund's first and second rules that describe electron arrangement.
Create the orbital notation for the element sulfur. Describe the orbital notation in detail. For example, 1s: up arrow down arrow; 2s up arrow down arrow; 2p three up arrows.
Answers
Explanation:
Hund's first and second rules describe how electrons are arranged in an atom's orbitals. Hund's first rule states that when electrons occupy orbitals of equal energy (such as the three p orbitals in a given shell), they will each first occupy separate orbitals before any orbital receives a second electron. This means that electrons will always try to maximize their spin, with one electron in each orbital having the same spin before any pairing occurs.
Hund's second rule states that if two or more orbitals of the same energy level are available, electrons will occupy empty orbitals before they pair up in an orbital that already has an electron.
Now, let's look at the orbital notation for sulfur. The atomic number of sulfur is 16, which means it has 16 electrons.
The orbital notation for sulfur would be:
1s² 2s² 2p⁶ 3s² 3p⁴
This indicates that sulfur has two electrons in the 1s orbital, two electrons in the 2s orbital, and six electrons in the 2p orbital, fully occupying all three 2p orbitals with two electrons in each and spinning in the same direction. Sulfur also has two electrons in the 3s orbital and four electrons in the 3p orbital, with a single electron in each of the three 3p orbitals and the fourth 3p orbital being half-filled. The half-filled 3p orbital is a consequence of Hund's rule, which predicts that electrons will fill each of the three 3p orbitals with one electron before any two orbitals receive a second electron.
Which image shows electronegativities of elements on the periodic table?

Answers
ANSWER
Fluorine
EXPLANATION
Based on the information provided, fluorine has the highest electronegativity and it form strong ionic bond with metals
Fluorine has 7 valence electron at its outermost shell and it readily to take on electron from its neighboring atom.
Therefore, the answer is fluorine