Na2CO3 + CaCl2•2H2O -> CaCO3 + 2NaCl + 2H2O
Calculate how many moles of CaCl2•2H2O are present in 1.50 g of CaCl2•2H2O and then calculate how many moles of pure CaCl2 are present in 1.50 g of CaCl2•2H2O. Then determine how many grams of Na2CO3 are necessary to reach stoichiometric quantities.
For CaCl2 I got 0.0135 mol but I have seen some put 0.0102 mol. Which is it?
For the initial mol of Na2CO3 I got 0.0102 mol but again I’m not sure if I’m right.
For the grams of Na2CO3 I got 1.08 g
Can someone help me figure out if I have this correct?
Answers
Answer:
See explanation
Explanation:
Number of moles = reacting mass/molar mass
Number of moles of CaCl2•2H2O = 1.50 g/147.02 = 0.0102 moles
From the equation;
Na2CO3 + CaCl2•2H2O -> CaCO3 + 2NaCl + 2H2O
We can see is 1:1
1 mole of Na2CO3 reacts with 1 mole of CaCl2•2H2O
x moles of Na2CO3 reacts with 0.0102 moles of CaCl2•2H2O
x = 1 × 0.0102 moles/1
x = 0.0102 moles of Na2CO3
Mass of Na2CO3 = 0.0102 moles of Na2CO3 × 106g/mol = 1.08 g of Na2CO3
Related Questions
Thermal decomposition of 5.00 metric tons of limestone to lime and carbon dioxide requires 9.00 × 106 kJ of heat. Convert this energy to (a) joules; (b) calories; (c) British thermal units. Give your answers in scientific notation.
Answers
Answer:
Take a look at the attachment below
Explanation:
Hope that helps!

how many molecules of potassium chloride will react if 21.89 grams KCl are added to the solution
Answers
There are approximately 1.765 x 10²³ molecules of KCl in 21.89 grams of KCl.
What is meant by potassium chloride ?Potassium chloride (KCl) is a compound made up of potassium and chloride ions. It is a colorless, odorless salt that is commonly used in a variety of applications.
Molar mass of KCl is 74.55 g/mol; number of moles = Mass/ Molar mass
So, the number of moles = 21.89 g ÷ 74.55 g/mol = 0.2936 mol
and the number of molecules = Number of moles * Avogadro's number
Number of molecules = 0.2936 mol x 6.02 x 10²³ molecules/mol
Number of molecules = 1.765 x 10²³ molecules
Therefore, there are approximately 1.765 x 10²³ molecules of KCl in 21.89 grams of KCl.
To know more about potassium chloride, refer
https://brainly.com/question/25380525
#SPJ1
benzen has a boiling point of 80.10 c we know the change in boiling point for a solution of c6h14 in benzen is 2.25 what is the new boiling point for the solution
bp= ? c
Answers
The pressure of the environment affects the liquid's boiling point. The boiling point of the liquid is higher when it is under high pressure than when it is under normal atmospheric pressure. For a given pressure, various liquids have different boiling points.
The temperature at which a liquid's vapour pressure equals the surrounding atmosphere is known as the boiling point of the liquid. This temperature causes the liquid to become a vapour.
The temperature of the liquid, the pressure of the atmosphere, and the pressure of the vapour all affect its boiling point.
We know that change in temperature of a system is given by the following formula:
Initial boiling point (T₁) = 80.1 °C
ΔT = T₂ - T₁
2.25 = T₂ - 80.1
T₂ = 82.35 °C
To know more about boiling point, visit;
https://brainly.com/question/28986258
#SPJ1
Answer:
82.35
Explanation:
acellus
PLEASE HELP!!!!
Why are dichotomous keys used?
A. They help with species identification
B. They help us identify everything from plants to minerals based on shared characteristics
C. They help organize a set of species or objects in a neat, easy to read diagram with branches
D. All of the above
Answers
the answer is D all of the above
(I will give a brainliest) What must be changed, temperature or heat during condensation?
Answers
Answer: Temperature
Explanation:
Answer: Temperature
Explanation: can i get brainliest plss
Question 2 (1 point)
What is the concentration when 45 mL of 1.5 M Na₂SO4 is diluted to 500 mL?
16.7 M
0.14 M
0.015 M
1.35 x 10-4 M
Answers
Answer: The concentration(molarity) of the solution will be 1.35 X10-4 M
Explanation: Molarity is defined as number of moles of solute present in one litre of any solution.
Mathematically,
Molarity= no of moles of solute/volume of solution(in liters)
M=N/V(liters)
Also upon dilution the number of moles of the solution remain unchanged
Therefore initial no of moles(before dilution)= final no of moles(after dilution)
M1V1=M2V2
According to the question,
Molarity of Na2S04 before dilution(M1)=1.5 M
Volume of NA2SO4(V1)=45 mL
Molarity of Na2S04 after dilution(M2)=x M
Volume of Na2S04(V2)=500 mL
x=M1V1/V2
=1.5X45/500
=0.135 M =1.35 X10-4 M
For dilution concept, refer-
https://brainly.in/question/11390951
How many atoms of phosphorus are in 8.80
mol of copper(II) phosphate?
Answers
By the concept of calculating moles ,it can br concluded that the no. of atoms of phosphorus in 8.80mol of copper(II) phosphate is=1.06\(x10^{25}\)
A mole is defined as amount of substance containing as many as elementary entities that are there in atoms of exactly 12 g of carbon-12.Therefore we can say,1 mole of copper(II) phosphate, Cu3(PO4)2, contains three moles of copper(II) cations and two moles of phosphate anions.
Again 1mole of phosphate anions contains one mole of phosphorus and four moles of oxygen.Considering all these informations we can conclude that:1 mole of copper(II) phosphate contains 2 moles of Phosphorus
Accordingle the sample contains=(8.80\(x\)2) moles of Phosphorus
=(17.6\(x\)Avogadro's constant) atoms of Phosphorus
=(17.6\(x\)6.022\(x\)\(10^{23}\)) atoms of Phosphorus
=1.06\(x10^{25}\) [approx] no. of Phosphorus atoms
to learn more about Moles,
https://brainly.com/question/30892840
In 8.80 moles of copper(II) phosphate, there are approximately 1.06 x 10²⁵ atoms of phosphorus.
Explanation:The number of atoms of phosphorus in a given amount of a compound can be calculated using the concept of mole in chemistry. Copper(II) phosphate is Cu3(PO4)2, containing 2 moles of phosphorus (P) for every 1 mole of the compound. Avogadro's number (6.022 x 10²³) gives the number of atoms in one mole.
So, if there are 8.80 moles of copper(II) phosphate, there would be 2 × 8.80 moles of phosphorus. Multiplying this by Avogadro's number gives the total number of phosphorus atoms.
Therefore, the number of phosphorus atoms is 2 × 8.80 × 6.022 x 10²³ = 1.06 x 10²⁵ atoms of phosphorus.
Learn more about Moles to atoms conversion here:https://brainly.com/question/32787463
#SPJ2
The radius of Pt atom in an fcc structure is 0.1386 nm. Pt has atomic mass of 195.09 g/mol. Calculate the density of this fcc structure. The Avogadro’s number NAis 6.022 x 1023 per mole
Answers
Answer:
21.51g/cm³
Explanation:
To answer this question we need to know that, in 1 unit FCC cell:
Edge length = √8 * R
Volume = 8√8 * R³
And there are 4 atoms per unit cell
The mass of the 4 atoms of the cell, in grams, is:
4 atom * (1mol / 6.022x10²³atom) * (195.09g / mol) = 1.2958x10⁻²¹g
Volume in cm³:
0.1386nm * (1x10⁻⁷cm / 1nm) = 1.386x10⁻⁸cm
Volume = 8√8 * (1.386x10⁻⁸cm)³
Volume = 6.02455x10⁻²³cm³
And density, the ratio of mass and volume, is:
1.2958x10⁻²¹g / 6.02455x10⁻²³cm³ =
21.51g/cm³
The IUPAC name of(CH3)2CHCH2CH=CH2
Answers
Answer:
4-Methyl-1-pentene
Explanation:
According to the International Union of Pure and Applied Chemistry (IUPAC), IUPAC nomenclature of organic chemistry is defined as the system of naming organic chemical compounds.
There are certain rules for naming organic chemical compounds as per IUPAC. The name of given (CH3)2CHCH2CH=CH2 will be based on the same rules.
At first find out the longest continuous chain and number the chain consecutively (preference given to double and single bonds), so numbering will start from right to left. the given chemical is 5 Carbon chain long.Then, identify the groups attached to this chain. Methyl is the only group attached to this chain at 4 Carbon.Assemble the name in alphabetical order using the full name using correct prefix and suffix.4-Methyl-1-pentene is the name of this chemical, as methyl is in 4th carbon position, Pent for 5 carbon chain and -ene (suffix) for one double bond).
Hence, the correct answer is "4-Methyl-1-pentene".
Look at the reaction below. Upper H subscript 2 upper S upper O subscript 4 (a q) plus upper Upper M g (s) right arrow Uper M g upper S upper O subscript 4 (a q) plus upper H subscript 2 (g). Which substance is the acid in the reaction? Mg(s) H2(g) MgSO4(aq) H2SO4(aq)
Answers
Answer:
H2SO4(aq)
Explanation:
The balanced equation for the reaction is given below:
H2SO4(aq) + Mg(s) —> MgSO4(aq) + H2 (g)
An acid is a substance which dissolves in water to produce hydrogen ion, H+ as the only positive ion.
To know which of the substance is acid, let us dissolve them in water to see which will produce hydrogen ion, H+ as the only positive ion.
This is illustrated below:
H2SO4(aq) —> 2H+(aq) + SO4^2-(aq)
Mg(s) + 2H2O(l) —> Mg(OH)2(aq) + H2(g)
MgSO4(aq) —> Mg^2+(aq) + SO4^2-(aq)
H2 is insoluble in water.
From the above, only H2SO4 produces hydrogen ion H+ on dissolution in water. Therefore, H2SO4 is an acid
Answer:
D on edg 2021
Explanation:
How many kilowatt-hours of energy are necessary to heat the air in the house from 9 ∘C to 27 ∘C? The heat capacity of air is 1.03 J/g∘C.
Answers
Answer:
The amount of air (in grams) is required for an answer. The process is outlined below.
Explanation:
An example for 1 kg of air:
Energy = (specific heat)*(mass)*(temperature change)
Joules = (1.03J/g°C)*(1,000g)*(27°C - 9°C)
Joules = 18540J
Coversion: 1 Joule = 2.78E-07 kwh
Kwh to heat 1 kg of air from 9 to 27°C is 5.15E-03 kwh
Use the same process with the actual mass of air in the house. If you are given house dimensions, calculate the volume and multiply by the density of air to get grams air. (Remember to subtract the volume taken up by household items (e.g., beer cans).
The kilowatt-hours of energy that are necessary to heat the air in the house from 9 ∘C to 27 ∘C is 5.15 x 10³.
What is specific heat capacity?When the temperature of a material rises 1 K (or 1 °C), the amount of heat (J) absorbed per unit mass (kg).
Given that, the heat capacity of air is 1.03 J/g∘C.
The changing temperature of the house is 9∘C to 27 ∘C
Here, the energy has to be calculated and the heat capacity is given. Then we have to change or convert the unit into kilowatt-hours.
Energy = (specific heat)*(mass)*(temperature change)
Joules = (1.03J/g°C) x (1,000g) x (27°C - 9°C)
Joules = 18540J
The conversion of the unit is 1 Joule = 2.78 x 10⁷
KWH to heat 1 kg of air from 9 to 27°C is 5.15 x 10³.
Thus, the energy that is necessary to heat the air in the house is 5.15 x 10³.
To learn more about specific heat capacity, refer to the link:
https://brainly.com/question/16801152
#SPJ2
If 4.02g of hydrogen and 32.00 of oxygen react completely to form water, what will be the mass of the water produced? Which scientific law dictates this?
Answers
Answer:
40.04g
Explanation:
the water has one molecule of hydrogen and two molecules of oxygen(H20)
(4.02)(2)(32)
Explain how to scale a life size crime scene onto a piece of sketch paper
Provide an example with correct calculations
also provide a citation
(ignore my tag where it says chem it's forensics science but ig this site doesn't have this tag)
Answers
Scaling a life-size crime scene onto a sketch paper involves reducing the dimensions of the scene while maintaining accurate proportions.
Here's an example of how to do it:
Measure the dimensions of the crime scene (e.g., length and width) using a tape measure.
Determine the desired scale for the sketch (e.g., 1 inch represents 1 foot).
Calculate the reduction factor by dividing the length of the crime scene by the length on the sketch paper. For example, if the crime scene length is 30 feet and the sketch length is 10 inches (120 inches), the reduction factor would be 30/120 = 0.25.
Multiply all measurements of the crime scene (length, width, objects, distances) by the reduction factor to obtain the corresponding measurements for the sketch.
Transfer the scaled measurements onto the sketch paper using a ruler and appropriate drawing tools.
Citation: The procedure described above is a commonly used method for scaling objects or scenes in forensic science investigations. It is based on principles of measurement and proportion commonly employed in the field. No specific citation is provided since this is a widely used technique in forensic science practice.
Therefore, scaling a life-size crime scene onto a sketch paper involves reducing the dimensions of the scene while maintaining accurate proportions.
for more such question on crime scene
https://brainly.com/question/25759350
#SPJ8
The rate constant for the reaction below was determined to be 3.241×10-5 s–1 at 800 K. The activation energy of the reaction is 255 kJ/mol. What would be the value of the rate constant at 9.30×102 K?
Answers
The value of the rate constant at 9.30×102 K is 2.03×102 s–1.
Rate constant from Arrhenius equation calculation.
To solve this problem, we can use the Arrhenius equation:
k = A * exp(-Ea/RT)
where:
k = rate constant
A = pre-exponential factor or frequency factor
Ea = activation energy
R = gas constant (8.314 J/mol·K)
T = temperature in Kelvin
We can rearrange this equation to solve for the rate constant at the new temperature:
k2 = A * exp(-Ea/RT2)
where k2 is the rate constant at the new temperature T2.
We are given k1 = 3.241×10-5 s–1 at T1 = 800 K, and Ea = 255 kJ/mol. We need to find k2 at T2 = 9.30×102 K.
To find A, we can rearrange the Arrhenius equation to solve for A:
A = k / exp(-Ea/RT)
Using k1 and T1, we get:
A = 3.241×10-5 s–1 / exp(-255000 J/mol / (8.314 J/mol·K * 800 K))
A = 7.07×1013 s–1
Now we can use A, Ea, k1, and T2 to find k2:
k2 = A * exp(-Ea/RT2)
k2 = 7.07×1013 s–1 * exp(-255000 J/mol / (8.314 J/mol·K * 930 K))
k2 = 2.03×102 s–1
Therefore, the value of the rate constant at 9.30×102 K is 2.03×102 s–1. using Arrhenius equation.
Learn more about Arrhenius equation below.
https://brainly.com/question/14739712
#SPJ1
A region of the IR spectrum can be used to differentiate between similar compounds that contain the same functional groups. Select the correct name of this region. O O O the downfield region the fingerprint region the functional group region the Ri region
Answers
The fingerprint region is the right term for this area. The IR spectrum of a molecule is a graphical depiction of the vibrational frequencies of the molecule's bonds.
The fingerprint area is the portion of the IR spectra between 1,000 and 400 cm-1 that is frequently used to distinguish between identical molecules with the same functional groups. This is due to the fact that each molecule has a unique bond configuration, which results in a unique set of vibrational frequencies.The fingerprint region is the right term for this area. The IR spectrum of a molecule is a graphical depiction of the vibrational frequencies of the molecule's bonds. It is possible to identify individual molecules and distinguish between similar ones by studying the peaks in the fingerprint area.The remaining areas indicated, the downfield, functional group, and Ri regions, are not typically used to define the IR spectrum.
learn more about IR spectrum here:
https://brainly.com/question/29753353
#SPJ4
thefunctionalgroupregion These are often connected to functional group stretching vibrationsA functional group's stretching vibrations are restricted to a small range
Functional connection is defined as the geographically dispersed neurophysiological events' temporal coherence (Friston, 1994). In other words, if there is a statistical link between the activity measurements that have been recorded for two areas, then those two regions are said to exhibit functional connectivity. This connection strategy is based on the idea that if two regions' functional activity is regularly associated, then those two areas are assumed to be connected or to be parts of the same network Functional connectivity is a considerably more direct method of analysing functional networks than effective connectivity studies, which rely on a number of assumptions about the underlying neurobiology and the model used to predict it. Particularly it is in agreement with the intuitive
Learn more about connected to functional here:
https://brainly.com/question/29739406
#SPJ4
3. Pluto picks up a snow globe and shakes it, he notices that the "snow" settles at the bottom after a few
seconds.
The snow globe can be classified as a:
a. suspension
b. element
c. compound
d. homogeneous mixture
Answers
A snow globe is a suspension combination because it shakes up with snow all over it, which eventually settles.
What is the crystal-clear substance in snow globes?Glycerin, a transparent liquid that is often manufactured from vegetable oils, is one of the secrets to making a snow globe. It is used frequently to reduce the impact of glitter and agitated water on the appearance of snowfall. Your snow globes risk drying out, cracking, and color fading if the right precautions aren't taken to protect them. In general, you should store your snow globes away from hot surroundings, bright sunlight, and other risky places where they could break.
To know more about suspension :
https://brainly.com/question/17650174
#SPJ9
Please help me!! I will literally pay you if you do my one aleks topic due tomorrow!! I have multiple example problems. It’s same Q different numbers
Answers
Answer:
I'll do it if you give brainlyest and some points
A gas has a volume of 550 mL at a temperature of -55 °C. The volume of the gas at 30 °C is
Blank 1:
mL.
Answers
The combined gas law equation is:
(P1 * V1) / (T1) = (P2 * V2) / (T2)
The volume of the gas at 30 °C is approximately 760.67 mL.
To determine the volume of the gas at 30 °C, we can use the combined gas law equation, which relates the initial and final conditions of temperature and volume for a gas.
The combined gas law equation is:
(P1 * V1) / (T1) = (P2 * V2) / (T2)
Where:
P1 and P2 are the initial and final pressures, respectively
V1 and V2 are the initial and final volumes, respectively
T1 and T2 are the initial and final temperatures in Kelvin, respectively
We need to convert the temperatures from Celsius to Kelvin by adding 273.15 to each value.
Given:
V1 = 550 mL
T1 = -55 °C = 218.15 K
T2 = 30 °C = 303.15 K
Assuming the pressure remains constant, we can rearrange the equation to solve for V2:
V2 = (P1 * V1 * T2) / (P2 * T1)
Since the pressure is not specified in the problem, we can assume it remains constant, allowing us to cancel out the pressure terms. Thus, the final equation becomes:
V2 = (V1 * T2) / T1
Plugging in the given values:
V2 = (550 mL * 303.15 K) / 218.15 K
Simplifying the calculation, we find:
V2 ≈ 760.67 mL
Therefore, the volume of the gas at 30 °C is approximately 760.67 mL.
For more question on gas law
https://brainly.com/question/27870704
#SPJ8
coenzyme q carries electrons between which stages of the electron-transport chain? check all that apply.
Answers
Coenzyme q carries electrons from complex I to complex III and complex II to complex III in the electron-transport chain.
Coenzyme q (CoQ), also known as ubiquinone, is the electron carrier in the electron transport system (ETS) present on the inner membrane of mitochondria.
Ubiquinone is a ubiquitous quinone, which accepts electrons from complex II ( succinate dehydrogenase) and reduces to ubiquinol ( reduced form)
The purpose of the ETS is to generate an H+ ion concentration, by carrying electrons obtained from NADH AND \(FADH_{2}\) produced by the Krebs cycle and glycolysis in the mitochondrial matrix. This H+ ion potential will be used by ATP synthase to generate ATP.
To know more about ETS :
https://brainly.com/question/876880
https://brainly.com/question/18686654
The inner mitochondrial membrane contains CoQ, a key part of the mitochondrial electron transport chain (ETC), which moves electrons from complexes I and II to complex III to provide energy for proton translocation to the intermembrane gap.
What is mitochondria ?An organelle called a mitochondrion can be found in the cells of the majority of Eukaryotes, including mammals, plants, and fungi. Adenosine triphosphate, which is produced by aerobic respiration in mitochondria with their double membrane structure, is used as a source of chemical energy throughout the entire cell.
Coenzyme Q10 takes electrons from reducing equivalents produced during the metabolism of fatty acids and glucose and then transports them to electron acceptors as part of the mitochondrial electron transport chain.
Ubiquinone, also known as coenzyme Q, is a lipophilic molecule that is found in all tissues and cells and is mostly found in the inner mitochondrial membrane. It is generally known that Coenzyme Q is an essential part of the oxidative phosphorylation process in mitochondria.
Thus, The inner mitochondrial membrane contains CoQ, a key part of the mitochondrial electron transport chain (ETC).
To learn more about mitochondria, follow the link;
https://brainly.com/question/10688306
#SPJ12
what happens to the volume of a gas if the pressure and temperature doubled
Answers
Hi there! Answer is below :)
Explanation:
For this question, we apply Boyle's Law and Charles' Law.
When you double the amount of pressure and temperature of a compound or mixture, the volume will decrease by a half. So, if the pressure and temperature are 2, and the volume is 1, and you double, your volume will be 0.5 and your pressure and temperature will be 4.
Best of Luck!
If you have 2m of hydrochloric acid and also 2m of vinegar which one is weak and strong and why?
Answers
Describe the “Doppler Effect.” Explain how it is used in Astronomy in term of red shift and blue shift
Answers
Answer:
This apparent change in the pitch (or frequency) of sound is called Doppler shift. ... You see these stretched-out light waves as having a lower frequency. Since red is at the low-frequency end of the visible spectrum, we say that light from a receding star is shifted toward red, or red shifted.
Explanation:
Water is a(n)______ molecule, and it easily dissolves _______ molecules.
Answers
How many atoms are in a 591 g sample of gold?
116,000 atoms
1.81 × 1024 atoms
3.00 atoms
3.60 × 1025 atoms
Answers
The number of atoms present in 591 g of gold is 1.81×10²⁴ atoms
Avogadro's hypothesisFrom Avogadro's hypothesis,
1 mole of Gold = 6.02×10²³ atoms
But
1 mole of gold = 197 g
Thus,
197 g of gold = 6.02×10²³ atoms
How to determine the atoms in 591 g of gold197 g of gold = 6.02×10²³ atoms
Therefore,
591 g of gold = (591 × 6.02×10²³) / 197
591 g of gold = 1.81×10²⁴ atoms
Thus, 1.81×10²⁴ atoms is present in 591 g of gold.
Learn more about Avogadro's number:
https://brainly.com/question/26141731
What is the formula of a compound if a sample of the compound contains 0.492 mol X, 0.197 mol Y, and 0.295 mol Z?
Answers
Answer:
X₅Y₂Z₃
Explanation:
The formula of a compound is determined as the whole number ratio between moles of each element present in the molecule.
The molecule is made from X, Y and Z. To fin the ratio we will divide the given moles in the moles of Y (0.197 moles), because is the element with the low number of moles.
X = 0.492 moles / 0.197 moles = 2.5
Y = 0.197 moles / 0.197 moles = 1
Z = 0.295 moles / 0.197 moles = 1.5
But, as the formula is given just with whole numbers, if we multiply each number twice:
X = 2.5*2 = 5
Y = 1*2 = 2
Z = 1.5*2 = 3
The formula is:
X₅Y₂Z₃Does the model represent a chemical reaction? (Image)
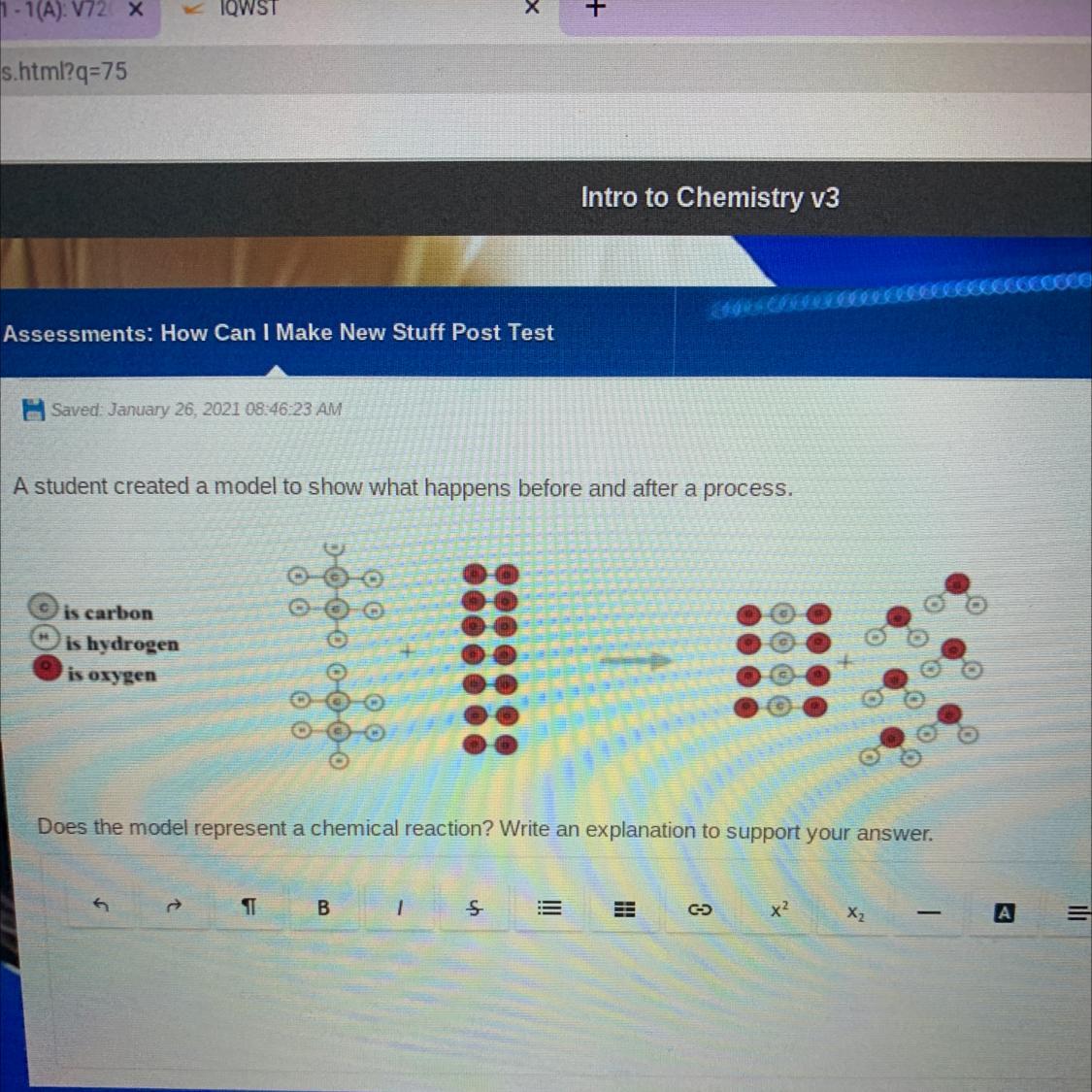
Answers
Answer:
yes it is a chemical reaction
Explanation:
because the substances combined and made something new
Indicate how CaCO3 neutralizes soil acidity. In other words, indicate how calcium carbonate chemically removes hydrogen from soil solution.
Answers
Answer:
Soil acidity can be corrected easily by liming the soil, or adding basic materials to neutralize the acid present. ... As lime dissolves in the soil, calcium (Ca) moves to the surface of soil particles, replacing the acidity. The acidity reacts with the carbonate (CO3) to form carbon dioxide (CO2) and water (H2O).
Explanation:
How do I go about solving a Nuclear Equation?

Answers
Answer:
How do amoeba respire.
How do plants respire.
Zeros between nonzero digits are significant
Answers
Answer:
Explanation:
If a zero is found between significant digits, it is significant
Which of the different components of smog depicted in the graph are most likely released from automobile exhaust?
A
A and B
B
B and C
с
A, B, and C
D
B, C, and D
Answers
Answer:
B, B and C
Explanation:
The two components (AA, hydrocarbons and BB, nitrogen oxide) are most likely released from automobile exhaust.
What is Smog?This is referred to a type of air pollution which could be from carbon emission etc and reduces the visibility.
(AA, hydrocarbons and BB, nitrogen oxide peaked midmorning when traffic is highest which means they were most likely released from automobile exhaust?.
Read more about Smog here https://brainly.com/question/14029972