Answers
The transition metal cations by their d electron count, with the highest at the top and the lowest at the bottom is A > E > B > C > D.
D8 Metals was founded in 2004 as a company specializing in aluminum and steel fabrication, architectural metals and claddings, and custom electromechanical projects. Instead of an 8-electron rule or octet, transition metals follow an 18-electron rule. The easiest way to count electrons is to break down the complex and count the electrons apart.
There are 40 elements in the d block of the periodic table. Four rows of transition elements make up the d block. The d-electron number is a chemical formalism used to describe the electronic configuration of the valence electrons of the transition metal centers of coordination complexes. The d electron number is an effective way to understand the shape and reactivity of transition metal complexes.
Learn more about Electron here:- https://brainly.com/question/14077813
#SPJ4
Related Questions
Omar wants to determine if the mass of a model rocket affects how long the rocket is able to stay up in the air. To do this, he constructs three identical rockets and then fills two of the rockets with varying amounts of sand to add mass. He then launches the rockets one at a time and times how long they are able to stay airborne.
What is the test variable (independent variable) in Omar's experiment?
A.
the materials out of which the rockets were made
B.
the time the rockets remain airborne
C.
the force with which each rocket is launched
D.
the masses of the model rockets
Answers
PLEASE HELP THIS IS DUE IN 10 MORE MINUTES I WILL GIVE YOU 30 POINTS ANSWER THE QUESTIONS BELOW AND THE QUESTION IN THE IMAGE!
1. Light always travels in a straight line until something gets in its way.
True or False
2. Waves carry energy. The amount of energy they carry is related to their frequency and their amplitude.
True or False
3.The higher the frequency of a wave, the more energy, and the higher the amplitude of a wave, the more energy.
True or False

Answers
It is true that Light always travels in a straight line until something gets in its way.
Waves do carry energy. The energy carried by a wave is directly related to both its frequency and amplitude. Higher frequency waves have more energy, and larger amplitude waves also carry more energy.
The frequency of a wave is related to its energy, but the amplitude of a wave is not directly proportional to its energy. The energy of a wave is determined solely by its frequency, not its amplitude.
The amplitude of a wave corresponds to its maximum displacement from the equilibrium position, while the energy of a wave is determined by the number of wave oscillations or cycles per unit time, which is represented by the frequency.
Thus, the answers are true, true and false respectively.
For more details regarding wave, visit:
https://brainly.com/question/25954805
#SPJ1
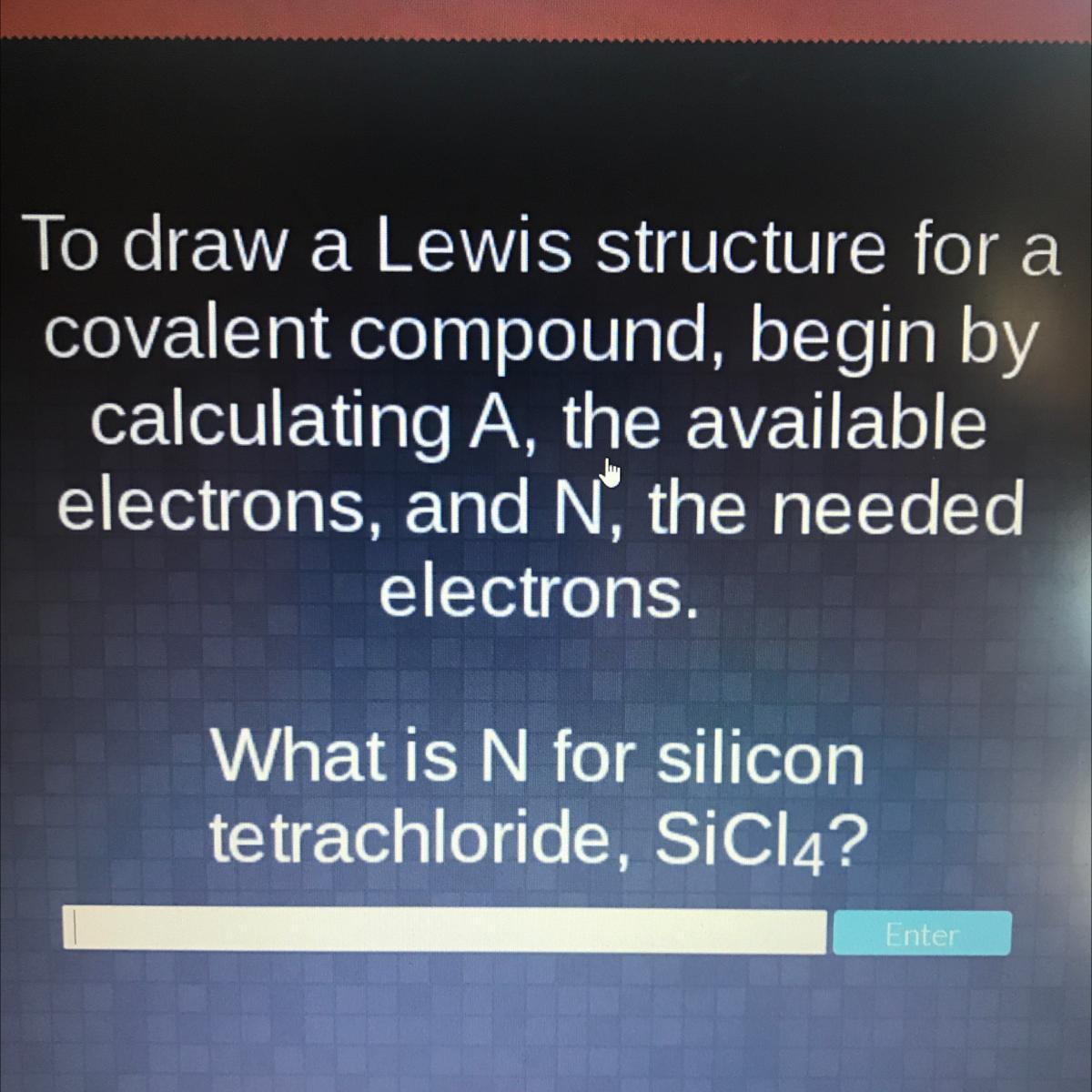
What is N for silicon tetrachloride, SiCl4?
Answers
How much heat is gained by nickel when 31.4 g of nickel is warmed from 27.2 °C to 64.2 °C? The specific heat of nickel is 0.443 J/g · °C.
Answers
Explanation:
To calculate the heat gained by nickel, we can use the formula:
q = m * c * ΔT
where q is the heat gained, m is the mass of the nickel, c is the specific heat of nickel, and ΔT is the change in temperature.
Given:
- Mass of nickel, m = 31.4 g
- Specific heat of nickel, c = 0.443 J/g · °C
- Change in temperature, ΔT = 64.2 °C - 27.2 °C = 37.0 °C
Substituting the values into the formula, we get:
q = (31.4 g) * (0.443 J/g · °C) * (37.0 °C)
Simplifying the calculation, we get:
q = 584 J
Therefore, the heat gained by nickel when 31.4 g of nickel is warmed from 27.2 °C to 64.2 °C is 584 J.
Compare and contrast the four different types of solar activity above the photosphere. (Select all that apply)
A. Prominences are huge loops of the Sun’s ionized but cool material that are pushed by magnetic forces from the chromosphere into the corona.
B. There are regions of higher density and temperature than the surrounding material in the chromosphere called plages.
C. Flares are long lived phenomena above the photosphere beginning at the solar minimum and ending at solar maximum.
D. Coronal mass ejections happen when a flare is so violent that the material exceeds the escape speed of the sun. It is connected to sunspots.
Answers
There are up to four different kinds of features that can be seen when viewing the photosphere. Sunspots, faculae, granulation, and super-granulation are listed in decreasing order of observational ease. All the given statement are true.
Solar activityIn the chromosphere, plateaus are areas that are more dense and hotter than the surrounding material. Large, chilly, ionized loops of material known as prominences are slowly pushed into the corona by magnetic energy from the chromosphere. Flares are erratic, fiery, and intensely energetic bursts that last only a moment or two. When a flare is extremely powerful, its material is thrown into the solar system beyond the Sun's escape velocity and causes coronal mass ejections. These are all essentially connected to sunspots.For more information on solar activity above photosphere kindly visit to
https://brainly.com/question/9053722
#SPJ1
three compounds that nitrogen and oxygen can form
Answers
Answer:
It's oxygen, nitrogen forms several oxides, including nitrous oxide.
Explanation:
Hope this helps you out for sure!
how many moles of glucose do I have if I have 26 grams of glucose
Answers
Answer:
0.1443194637888042 moles
Explanation:
Hope This Helps
Have A Great Day
~Zero~
If you dilute 175 ml of a 1.6 M solution of LiCl to 1.0 L , determine the new concentration of the solution
Answers
1) List the known quantities
Initial conditions
Concentration 1: 1.6 M
Volume 1: 175 mL
Final conditions
Volume 2: 1 L
Concentration 2: unknown
2) Set the equation
\(C1*V1=C2*V2\)3) Convert units
Volume
1 L = 1000 mL
\(mL=1\text{ }L*\frac{1000\text{ }mL}{1\text{ }L}=1000\text{ }mL\)4) Plug in the known quantities and solve for C2
\((1.6\text{ }M)*(175\text{ }mL)=C2*(1000\text{ }mL)\)\(C2=\frac{(1.6\text{ }M)*(175\text{ }mL)}{(1000\text{ }mL)}\)\(C2=0.28\text{ }M\)
The new concentration is 0.28 M.
.
Balance this equation.
_Mg +_Cl2 -->_MgCl2
Answers
Answer:
1Mg + 1Cl2 = 1MgCl2
Explanation:
Hi! When balancing an equation, you want to make sure that there are equal amounts of each element on both sides. When looking at the equation that you provided in the question, look and count how many of each element are on each side. I can see that there is 1 Mg ion on the left and 1 Mg ion on the right. There are also 2 Cl ions on the left and 2 Cl ions on the right. Because they are already equal, the coefficients in front of each compound will be 1. If the amounts were different on each side, that is when you would need to add different coefficients.
Hope this helps! Let me know if you have any other questions about this!
871g of sodium chloride is how many moles
Answers
Answer:
14.9 mol
Explanation:
To find the number of moles in a given mass of a sample of sodium chloride (NaCl), we can multiply the number of grams in the sample by the molar mass of sodium chloride, which is 58.44 g/mol.
871 g × (1 mol / 58.44 g)
= 871/58.44 mol
≈ 14.9 mol
Note that we rounded to 3 significant figures in the final answer because that is how many significant figures were given in the mass measurement of the sodium chloride sample.
Mass is measured in= a. Liters b. centimeters c. newtons d. kilograms
Answers
10. A sample of an unknown composition was tested in a laboratory. The sample could not be broken down by
physical or chemical means. On the basis of these results, the laboratory reported that the unknown sample was
most likely
A. a compound
B. an element
C. a mixture
Answers
Answer:
it would be an element because its an element
Explanation:
KF + 02
Balance the equation
Answers
Explanation:
this equation is balanced
if you look at it carefully
k=1
f=1
o=2
we do not have any opposing element
Answer:
this equation is balanced
if you look at it carefully
k=1
f=1
o=2
we do not have any opposing element
What is a quasar?
Two stars moving around each other.
A star that emits a repeated radio signal.
A star that emits intense radio and light energy.
A system of stars held together by gravity.
A collapsed star emitting no light.
Answers
A quasar is a star that emits intense radio and light energy.
A quasar is an extremely bright radio source. It is called a quasi-stellar radio source. It appears to be like a star but is not a star. These quasars are young galaxies that are located far away from us and are highly luminous. The luminosity of a quasar is 1000 times greater than the luminosity of a milky way galaxy.
A quasar is powered by a supermassive black hole with its mass ranging from millions to tens of billions of solar masses, surrounded by a gaseous accretion disc.
The quasars were first discovered in the 1950s using the Hubble space telescope and were found to be a massive bright source emitting radio waves of unknown origin. But now, millions of quasars were discovered.
To know more about stars, click below:
https://brainly.com/question/1041175
#SPJ1
50 POINTS! pls answer


Answers
Answer:
reach us for assistance https://toplivewriters.com
Explanation:
I will give 20 points for correct answer please.
A graduated cylinder is filled to 20.0 mL with water and a piece of glass is placed in the cylinder, displacing the level to 36.4 mL. what is the volume of the glass piece in cubic centimeters?

Answers
The volume of the glass piece in cubic centimeters is 16.4 cm3
Volume
When adding an object to a liquid, the displayed volume difference corresponds to the volume of the added object.
In this way, to find the value of the object's volume, subtract the initial volume from the final volume:
\(36.4ml - 20ml\)
\(=16.4ml\)
ConversionTo convert to cubic centimeters, the conversion scale of volume and length measurements need to be used, finding that mL is equal to cubic centimeters. So, the final answer is:
\(=16.4cm^{3}\)
Learn more about units conversion and volume: brainly.com/question/15163977
Answer:
16.4
Explanation:
Scientific theories are based on evidence.....
True
False
Answers
Answer:
True
Explanation:
if not you can't support it
Describe how naturally
acidic rainwater can affect a
mountain of limestone.
Answers
Answer:
The naturally acidic rain will gradually wear the mountain of limestone away.
Hope it helps!
16.
Which chemical reaction will always be spontaneous? (1 point)
an endothermic reaction in which
entropy increases
an exothermic reaction in which
entropy increases
an endothermic reaction in which
entropy decreases
an exothermic reaction in which
entropy decreases
Answers
Answer: an exothermic reaction in which entropy increases
Explanation:
Spontaneous reactions normally cause an increase in entropy and are also exothermic.
how may liters are in 0.8291moles of hexane (c6h14)?
Answers
In 0.8291 moles of hexane (\(C_6H_1_4\)) there are 20.8 liters in 0.8291 moles of hexane at room temperature and atmospheric pressure.
To determine the number of liters in 0.8291 moles of hexane (C6H14), we need to use the ideal gas law equation:
PV = nRT
where:
P = pressure (atm)
V = volume (L)
n = moles of gas
R = gas constant (0.0821 L*atm/mol*K)
T = temperature (K)
We need to rearrange this equation to solve for V:
V = nRT/P
First, we need to calculate the number of moles of hexane:
n = mass/molar mass
The molar mass of hexane (C6H14) is:
6(12.01 g/mol) + 14(1.01 g/mol) = 86.18 g/mol
n = 0.8291 moles
Next, we need to convert the temperature to Kelvin. Assuming room temperature (25°C or 298 K):
T = 298 K
Finally, we need to assume a pressure value. Let's assume atmospheric pressure (1 atm).
P = 1 atm
Now we can plug in the values and solve for V:
V = (0.8291 mol)(0.0821 L*atm/mol*K)(298 K)/(1 atm)
V = 20.8 L
Therefore, there are 20.8 liters in 0.8291 moles of hexane at room temperature and atmospheric pressure.
For more details regarding ideal gas law, visit:
https://brainly.com/question/28257995
#SPJ1
Density involves the amount of a material in a certain volume. When a material changes phases, it changes in density in a predictable way as the amount of material stays the same but the molecules get farther apart or closer together. Water has solid and liquid states that do not follow these predictions of density in the phases of matter. What does that mean about the densities of the phases of water? The solid state is the most dense, followed by the liquid state, then the gas state. The solid state is more dense than the liquid state. The liquid state is more dense than the solid state. The gas state is the most dense, followed by the liquid state, then the solid state.
Answers
The liquid state of water is more dense than the solid state; option C.
What is density?Density is the mass per unit volume of a substance.
Density = mass/volume
The density of a given mass of water in the solid, liquid and gaseous state can be described thus:
The liquid state is most dense as it occupies the least volume.The solid state is less dense than the liquid stateThe gaseous state is least dense.Learn more about density of water at: https://brainly.com/question/20690558
#SPJ1
Answer: c- the liquid state is more dense than the solid state.
Explanation:

Someone help me I don’t know

Answers
Answer:
What's the gas given in the question??
3. A light bulb containing argon gas is switched on,
and after 30 minutes its temperature is 418 K
What Celsius temperature is equal to 418 K?
Answers
Answer:
The assumption is quite reasonable.........
A lightbulb contains Ar gas at a temperature of 295K and at a pressure of 75kPa. The light bulb is switched on, and after 30 minutes its temperature is 418 K. What is a numerical setup for calculating the pressure of the gas inside the light bulb at 418K?
Explanation:
P
1
T
1
=
P
2
T
2
given constant
n
, and constant
V
, conditions that certainly obtain with a fixed volume light bulb.
And so
P
2
=
P
1
T
1
×
T
2
=
75
⋅
k
P
a
295
⋅
K
×
418
⋅
K
≅
100
⋅
k
P
a
.
Had the light bulb been sealed at normal pressure during its manufacture, what do you think might occur when it is operated?
6) The density of ammonia gas (NHs) in a 6.0 L container at a pressure of 820 mm Hg and a g/L.
Answers
The density of ammonia gas in the 6.0 L container at a pressure of 820 mm Hg is approximately 0.805 g/L.
To determine the density of ammonia gas (NH3) in a 6.0 L container at a pressure of 820 mm Hg, we need to use the ideal gas law equation, which relates pressure, volume, number of moles, and temperature for a given gas.
The ideal gas law equation is:
PV = nRT
Where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
Since we are given the pressure (820 mm Hg), volume (6.0 L), and assuming standard temperature and pressure (STP), we can use the values for R (0.0821 L·atm/(mol·K)) and convert the pressure to atm by dividing by 760 (1 atm = 760 mm Hg).
820 mm Hg / 760 mm Hg/atm = 1.08 atm
Now we can rearrange the ideal gas law equation to solve for density (d):
d = (P * M) / (RT)
Where M is the molar mass of ammonia (NH3), which is approximately 17.03 g/mol.
Substituting the values, we have:
d = (1.08 atm * 17.03 g/mol) / (0.0821 L·atm/(mol·K) * 298 K)
Simplifying the equation, we find:
d ≈ 0.805 g/L
Therefore, the density of ammonia gas in the 6.0 L container at a pressure of 820 mm Hg is approximately 0.805 g/L.
For more question on density
https://brainly.com/question/26364788
#SPJ8
Two asteroids are 75,000 m apart one has a mass of 8 x 10^7 N what is the mass of the other asteroid
Answers
The mass of the asteroid is C. 1.2 x \(10^{12}\) Kg
To find the mass of the other asteroid, we can rearrange the equation for the gravitational force between two objects:
F = (G * m1 * m2) / \(r^{2}\)
where F is the force of gravity, G is the gravitational constant, m1 and m2 are the masses of the two asteroids, and r is the distance between them.
Given that the distance between the asteroids is 75000 m, the force of gravity between them is 1.14 N, and one asteroid has a mass of 8 x \(10^{7}\) kg, we can substitute these values into the equation and solve for the mass of the other asteroid (m2):
1.14 N = (6.67430 × \(10^{-11}\) N \(m^{2}\)/\(Kg^{2}\) * 8 x \(10^{7}\) kg * \(m2\)) / \((75000 m)^{2}\)
Simplifying and solving the equation, we find that the mass of the other asteroid (m2) is approximately 1.2 x \(10^{12}\) kg. Therefore, Option C is correct.
The question was incomplete. find the full content below:
Two asteroids are 75000 m apart one has a mass of 8 x \(10^{7}\) kg if the force of gravity between them is 1.14 what is the mass of the asteroid
A. 3.4 x \(10^{11}\) kg
B. 8.3 x \(10^{12}\) kg
C. 1.2 x \(10^{12}\) kg
D. 1.2 x \(10^{10}\) kg
Know more about gravitational force here:
https://brainly.com/question/72250
#SPJ8
What are measures of dispersion?
Answers
125 grams of zinc was reacted. What volume liters of hydrogen, measured at STP was released?
Answers
125 grams of zinc was reacted, therefore, 125 grams of zinc will produce 42.6 liters of hydrogen gas at STP, and the problem involves stoichiometry, which is the calculation of reactants and products in a chemical reaction.
The balanced chemical equation for the reaction of zinc and hydrochloric acid is:
Zn + 2HCl → ZnCl₂ + H₂
From the equation,
125 g Zn x (1 mole Zn/65.38 g) = 1.91 moles Zn
So, one can expect 1.91 moles of hydrogen gas to be produced.
Now, at STP (standard temperature and pressure), the volume of one mole of gas is 22.4 liters. Therefore, the volume of hydrogen gas produced at STP can be calculated as:
1.91 moles H2 x 22.4 L/mole = 42.6 L H2
Therefore, 125 grams of zinc will produce 42.6 liters of hydrogen gas at STP.
Learn more about the ideal gas law here.
https://brainly.com/question/30556543
#SPJ1
c55h72mdn4o5 what is the molar mass
Answers
If we sum the molar masses of the elements, we find that the molar mass of the compound C₅₅H₇₂N₄O₅ is 869.31 g/mol.
What is the molar mass?The molar mass is the mass in grams of 1 mole of a substance.
To calculate the molar mass of a compound, we need to sum the molar masses of the elements that form it, which can be found in the periodic table.
M(C₅₅H₇₂N₄O₅) = 55 M(C) + 72 M(H) + 4 M(N) + 5 (O)
M(C₅₅H₇₂N₄O₅) = 55 (12.01 g/mol) + 72 (1.01 g/mol) + 4 (14.01 g/mol) + 5 (16.00 g/mol) = 869.31 g/mol
There is a mistake in the question. The original question is:
C₅₅H₇₂N₄O₅, what is the molar mass?
Learn more about molar mass here: https://brainly.com/question/21334167
#SPJ1
What kind of fault movement will create a tsunami?

Answers
Answer:
I THINK IT'S BECAUSE THE EARTH UNDER THE SEA SHIFTS AND THE VIBRATION OF THE WATER GETS BIGGER UNTIL IT BECOMES A TSUNAMI. BRAINLIST ???
Green plants absorb sunlight to power photosynthesis, the chemical synthesis of food from water and carbon dioxide. The compound responsible for light absorption and the color of plants, chlorophyll, strongly absorbs light with a wavelength of 430.nm. Calculate the frequency of this light. Round your answer to 3 significant digits.
Answers
Answer:
\(698 \ THz\)
Explanation:
Data provided as per the question below:-
Wavelength = 430.nm
The computation of the frequency of the light is shown below:-
Frequency = Velocity of light ÷ Wavelength
The Velocity of light = \(= {3.0 \times 10^8 \ m/s}\)
Wavelength = 430 nm = \(4.30 \times 10^-^7\) m
Frequency = \(\frac{3.0 \times 10^8 \ m/s}{4.30 \times 10^-^7}\)
\(= 6.98 \times 10^1^4 \ hz\)
\(= 6.98 \times 10^2 \ Thz\)
= \(698 \ THz\)
Therefore for determining the frequency we simply applied the above formula.