lipids that contain a high number of double bonds in their fatty acid chains will ________.
Answers
Lipids that contain a high number of double bonds in their fatty acid chains will have a lower melting point.
What is bond?Bond is a type of debt security in which an investor loans capital to an issuer in exchange for a fixed rate of interest paid over the life of the bond. Bonds are typically issued by governments, corporations, and other organizations seeking to raise money and are often used to finance public and private projects. Bond investments are attractive because they generally provide a steady and reliable stream of income, and are considered to be a relatively low risk investment compared to stocks. Bonds can also be used to diversify a portfolio, and allow investors to gain exposure to different sectors and industries.
To learn more about bond
https://brainly.com/question/819068
#SPJ1
Related Questions
Which of the following would not be considered matter?
clouds
trees
rain
air
light
Answers
Answer:
light
Explanation:
Have a nice day
Answer:
light
Explanation:
PLEASE HELP RN ITS URGENT

Answers
Answer: transporting oxygen-rich blood to the body
Explanation:
How many moles of ammonium nitrate are in 335 mL of 0.425 M NH4NO3?
Answers
Answer:
0.142
Explanation:
Answer:
0.142 moles.
You would have to convert the algorithm to a broken down version of the compound.
NH4NO3(aq] → NH+4(aq] + NO−3(aq]
The ammonium is removed for this case, concentration gets broken down to zero.
So here, if 1 liter is equal to 10 x 10 x 10 milliliters...
use the formula of n and c to help you figure out the moles.
C is equal to N divided by V, so in that case...
N equals C times V.
C times V is 0.425, or 17 over 40 in simplest form.
From there, multiply 17 over 40 by the current moles, cross out the liters, and multiply by 335, then, 10 to the power of -3. You have to flip it to negative, because it will cancel out either way. Change the equation by the liters, and you have 0.125
Hence, your correct answer is 0.125
2h2 + 02 = 2h20
what is the volume of steam could be produced at stp if 12.8 g of oxygen reacts with excess hydrogen
Answers
The volume of steam that could be produced at STP if 12.8 g of oxygen reacts with excess hydrogen is 17.92 L
How to determine the volumeWe'll begin by obtaining the mole of steam produced. This can be obtained as follow:
Mass of O₂ = 12.8 gMolar mass of O₂ = 2 × 16 = 32 g/molMole of O₂ = 12.8 / 32 = 0.4 mole2H₂ + O₂ -> 2H₂O
From the balanced equation above,
1 mole O₂ reacted to produce 2 moles of steam, H₂O
Therefore,
0.4 mole O₂ will react to produce = 0.4 × 2 = 0.8 mole of steam, H₂O
Finally, we shall determin the volume as follow:
At standard temperature and pressure (STP),
1 mole of H₂O = 22.4 L
Therefore,
0.8 moles of H₂O = (0.8 mole × 22.4 L) / 1 mole
0.8 moles of H₂O = 17.92 L
Thus, we can conclude that the volume produced is 17.92 L
Learn more about volumes at STP:
https://brainly.com/question/22311771
#SPJ1
The fuel used to power the booster rockets on space shuttles is a mixture of aluminum metal and ammonium perchlorate. the following balanced equation represents the reaction.. 3al 3nh4clo4 → al2o3 alcl3 3no 6h2o how many moles of water are produced from 373 mol al?
Answers
The moles of water that are produced from 373 moles of ammonia present in any chemical reaction is 746 moles.
What is the stoichiometry?Stoichiometry of the reaction gives idea about the amount of entities present in any chemical reaction before and after the reaction.
Given chemical reaction is:
3Al + 3NH₄ClO₄ → Al₂O₃ + AlCl₃ + 3NO + 6H₂O
From the stoichiometry of the reaction it is clear that:
3 moles of Al = produces 6 moles of H₂O
373 moles of Al = produces 6/3×373 = 746 moles of H₂O
Hence required moles of water is 746 moles.
To know more about stoichiometry, visit the below link:
https://brainly.com/question/21931988
why are properties of covalent compounds so diverse
Answers
Answer:
The diversity of physical properties among covalent compounds is mainly because of widely varying intermolecular attractions. ... All of the atoms are covalently bonded to each other.
Evaluate importance:
- Why does a patch of garbage in the ocean effect humans on land?
Answers
Why does a patch of garbage in the ocean effect humans on land
This is the last one I need. Just want to make sure I did it right.

Answers
To combine ions to form ionic compounds, we need the combine in such a way that it gets neutral charge.
We can combine each anion with each cation to get the 4 compounds we need.
To combine SO₄²⁻ with Pb⁴⁺ we first find the Least Common Multiple of their charges, 2 and 4.
They have the factor 2 in common, so the LCM is 4. This is the final charge of each that will cancel out.
To get 4+, we only need 1 Pb⁴⁺.
To get 4-, we need 2 SO₄²⁻.
So, the formula is:
Pb(SO₄)₂
To combine SO₄²⁻ with NH₄⁺ is easier because one of them has single charge. In this case, we can simply pick one of the multiple charge ion and the same amount that will cancel its charge of the single charged one.
So, we picke 1 SO₄²⁻, ending with 2-.
And we picke 2 NH₄⁺, ending with 2+.
The formula:
(NH₄)₂SO₄
To combine C₂H₃O₂⁻ with Pb⁴⁺ we do the same, because the anion is single charged.
Pick 1 Pb⁴⁺, ending with 4+.
Pick 4 C₂H₃O₂⁻, ending with 4-.
The formula:
Pb(C₂H₃O₂)₄
To combine C₂H₃O₂⁻ with NH₄⁺, both have same charge, so we just need one of each and their charges will cancel out.
The formula:
NH₄C₂H₃O₂
So, the formulas are:
Pb(SO₄)₂
(NH₄)₂SO₄
Pb(C₂H₃O₂)₄
NH₄C₂H₃O₂
What forms when two atoms combine? PLEASE HELP!!!!!!!
A. a molecule
B. a nucleus
C. a proton
D. an electron
Answers
Answer:
Option A - nucleus
Explanation:
A molecule is formed when two or more atoms join together chemically. If atoms combine that are of two or more different elements, we call that a compound. All compounds are molecules, but not all molecules are compounds.
Two atoms combine to form a molecule.
What is a molecule?
Molecule is two or more atoms connected by chemical bonds and form the smallest unit of a substance that retains the composition and properties of that substanceThe atoms can be the same like in oxygen molecule - An oxygen molecule has 2 oxygen atomsThe atoms can be different like in a water molecule - A water molecule has 2 hydrogen and 1 oxygen atomWhat is a nucleus?
The nucleus is positively charged region at the center of the atomIt consists of two types of subatomic particles packed together. The particles are protons and neutronsProton has a positive charge and neutron is electrically neutralWhat is a proton?
A proton is a subatomic particle found in nucleus of every atomProtons have a positive electric charge The number of protons in each atom is its atomic numberWhat is an electron?
It was discovered by English physicist J.J.Thomson Electron is a negatively charged subatomic particle that can be either bound to an atom or freeThey exist outside the nucleusThey are significantly smaller in mass and have both wave-like and particle-like characteristicsLearn more about Molecules at https://brainly.com/question/13348791
#SPJ2
What is the name of Pb(NO3)2? Explain how you determined the bond type and the steps you used to determine the naming convention for the compound.
Answers
This chemical is known as lead (II) nitrate. It is an ionic assembly (salt compound) comprised of lead cations in the +2 oxidation state. With regard to the naming convention, each lead (II) cation is paired with two nitrate anions, each having a charge of -1.
What is a naming convention in Chemistry?Chemical nomenclature is a set of principles for naming chemical substances in a systematic manner. The International Union of Pure and Applied Chemistry designed and developed the most widely used nomenclature in the world (IUPAC).
The basic goal of chemical nomenclature is to guarantee that no ambiguity exists between a spoken or written chemical name and the chemical compound to which the name refers. Each chemical name should only relate to one substance.
It is required to indicate the charge of these cations or compounds containing these cations when identifying them. Ionic compounds are formed when cations and anions interact. The cation of an ionic compound is named first, followed by the anion. When writing their chemical formulae, they use the same format.
Learn more about naming conventions:
https://brainly.com/question/14326884
#SPJ1
Describe how spead, velocity and
brcoloration are related.
Answers
Answer:
Speed, being a scalar quantity, is the rate at which an object covers distance. The average speed is the distance (a scalar quantity) per time ratio. On the other hand, velocity is a vector quantity; it is direction-aware. Velocity is the rate at which the position changes.
The air in the balloon i heated up by leaving it in a warm place. Give two effect that thi ha on the air particle
Answers
If the balloon is closed, then yes, both volume and pressure will increase when the gas inside is heated.
What is pressure?
Pressure is the force applied perpendicular to the surface of an object per unit area over which that force is distributed.
Various units are used to express pressure. Some of these are units of force divided by units of area. For example, the SI unit of pressure, Pascal (Pa), is 1 Newton per square meter (N/m2). Similarly, pounds force per square inch (psi, symbol lbf/in2) is the traditional unit of pressure in imperial and US systems. Pressure can also be expressed as standard atmospheric pressure. Atmospheric pressure (atm) is equal to this pressure and torr is defined as 1/760 of this. Manometric units such as centimeters of water, millimeters of mercury, and inches of mercury are used to express pressure as the height of a particular liquid column within a manometer.
If the balloon is closed, then yes, both volume and pressure will increase when the gas inside is heated.
To know more about Pressure, visit:
https://brainly.com/question/28012687
#SPJ4
What is the main impurity that can form if you allow your reaction mixture to warm up too much during the addition of the benzophenone?
A Triphenylmethane
B. Benzene
C. Biphenyl
D. None of the above
Answers
The main impurity that can form if the reaction mixture is allowed to warm up too much during the addition of benzophenone is (C) biphenyl. The correct option is C.
Benzophenone, when exposed to excessive heat, can undergo a side reaction called the benzoin condensation.
This condensation reaction involves the coupling of two benzophenone molecules to form a molecule of biphenyl.
The reaction is catalyzed by base and occurs through the generation of a benzophenone anion followed by a nucleophilic attack on another benzophenone molecule.
If the reaction mixture becomes too hot during the addition of benzophenone, it can promote the formation of biphenyl as an impurity alongside the desired product.
Biphenyl is a byproduct of the benzoin condensation reaction and can negatively affect the purity and yield of the desired compound. Therefore, it is important to control the temperature during the reaction to minimize the formation of biphenyl.
To know more about benzophenone, refer here:
https://brainly.com/question/31957356#
#SPJ11
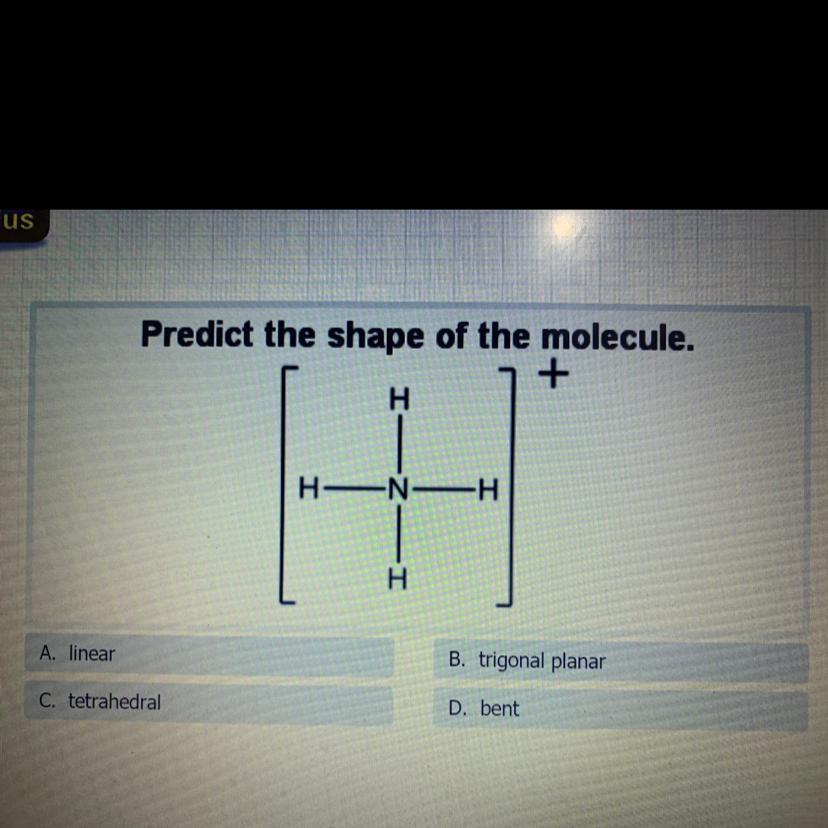
Predict the shape of the molecule.
B. trigonal planar
A. linear
D. bent
C. tetrahedra
Answers
Which image represents the step in mitosis when chromosomes condense and spindle fibers form?




Answers
Answer:
The answer is A
Explanation:
I did the unit test review
gallium has two naturally occurring isotopes, and . if has an atomic mass of 68.926 amu, has an atomic mass of 70.925 amu, and the atomic mass of gallium is 69.723 amu, what is the percent abundance of ?
Answers
If has an atomic mass of 68.926 amu, has an atomic mass of 70.925 amu, and the atomic mass of gallium is 69.723 amu, the percent abundance of 70Ga is 101.68%.
To find the percent abundance of an isotope, you need to determine its relative abundance compared to the total amount of the isotopes present. Here's how you can do it for the isotope 70Ga:
Calculate the fraction of 70Ga in the sample:
fraction of 70Ga = (mass of 70Ga) / (atomic mass of Gallium)
fraction of 70Ga = (70.925 amu) / (69.723 amu)
fraction of 70Ga = 1.0168
Convert the fraction to a percentage:
percentage of 70Ga = (fraction of 70Ga) * 100
percentage of 70Ga = (1.0168) * 100
percentage of 70Ga = 101.68%
To know more about isotope
https://brainly.com/question/11680817
#SPJ4
what are plasmas properties?
Answers
Answer:Plasma is highest energy state of matter.It consists of electrons,protons and neutral particles.
Explanation:(1) Plasma has a very high electrical conductivity .
(2) The motion of electrons and ions in plasma produces it's own electric and magnetic field
(3)It is readily influenced by electric and magnetic fields .
(4)It produces it's on electromagnetic radiations.
The endoplasmic reticulum and the golgi complex look a lot alike in both
plant and animal cells. Explain the difference in their functions.
Answers
Answer:
Explanation:
The Golgi apparatus receives proteins and lipids (fats) from the rough endoplasmic reticulum. It modifies some of them and sorts, concentrates and packs them into sealed droplets called vesicles.
Place the
elements in order from low number of valence electrons to high number of valence electrons.
1
Neon
2
Aluminum
3
Silicon
4.
Potassium
5
Magnesium
Answers
what is relative abundance isotopes
Answers
The relative abundance of isotopes is the number of atoms of a particular isotope divide by the total number of atoms of all isotopes of that element, multiplied 100 percent.
What is relative abundance isotopes?The relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring sample of an element.
Also relative abundances refers to the relative proportions of the stable isotopes of each element. They are most often quoted as atom percentages
To calculate the percent abundance of each isotope in a sample of an element, the number of atoms of a particular isotope is usually divide by the total number of atoms of all isotopes of that element and then multiply the result by 100 since it is expressed in percentage.
Mathematically, the formula for relative abundance is given as;
R.A = ( number of atoms of isotope / total number of atoms ) x 100%
Learn more about relative abundance here: https://brainly.com/question/6844925
#SPJ1
What are the missing coefficients for the skeleton equation below?
Al₂(SO4)3(aq) + KOH(aq) Al(OH)₂(aq) + K₂SO4(ag)
a. 1,3,2,3
b. 2, 12, 4, 6
c. 4,6,2,3
d. 1,6,2,3
X
Answers
A simplified description of a chemical reaction known as a "skeletal equation" contains only the chemical formulas of the reactants and products. It is devoid of details regarding relative quantities or equilibrium equation.
The following coefficients are missing from the skeleton equation:
Al₂(SO₄)₃(aq) + 6KOH(aq) → 2Al(OH)₂(aq) + 3K₂SO₄(aq)
Chemical equations are often balanced by beginning with the skeletal equation. The coefficients (the numbers in front of the formulas) are then changed to ensure that the number of atoms of each element is balanced on both sides of the equation. The stoichiometry and ratio of reactants and products in a chemical reaction is given by a balanced equation.
Learn more about skeletal equation, here:
https://brainly.com/question/29183611
#SPJ1
How do metals bond with each other
Answers
Answer:
Metallic bonding
Metals have low ionization energies. Therefore, their valence electrons are easily delocalized (attracted to the neighbouring metal atoms). These delocalized electrons are then not associated with a specific metal atom. Since the electrons are “free”, the metal atoms have become cations, and the electrons are free to move throughout the whole crystalline structure.
We say that a metal consists of an array of cations immersed in a sea of electrons. The electrons act as a “glue” holding the cations together.
Metallic bonds are the attractive forces between the metal cations and the sea of electrons.
(Hope this helps) Sky
Answer:
i dont knowi dont know and thank you for the points
Explanation:
Quartzite is a coarse-grained rock derived from sandstone.
Which type of rock is quartzite?
metamorphic
extrusive
sedimentary
igneous
Answers
Answer:
granoblastic metamorphic rock
Explanation:
Why were your results for the Southern Hemisphere opposite of what you found for
the Northern Hemisphere?
Answers
why water can reduce unpleasant smell that produce from methane gas ?
Answers
Answer:Water contains Oxygen the purified the methane gas
Explanation:
Which hypothesis of Thomson was later found to be not true by Rutherford and why?
Answers
Answer:
Explanation:
The electrons revolved around the nucleus, like the rings revolving around Saturn. In 1911, Rutherford showed that Thomson's model was "wrong": the distribution of positive and negative particles was not uniform. Rutherford showed that the atom contains a small, massive, positively charged nucleus.
The Thomson's atomic model is also called the plum pudding model. His atomic model was discarded after the discovery of Rutherford's model of atom.
What is Thomson's atomic model?According to Thomson's atomic model, an atom is considered as a uniform sphere of positive electricity with electrons embedded throughout which gives the most stable arrangement like raisins scattered in a plum pudding.
Rutherford showed that Thomson's model is wrong, because the distribution of positive and negative particles was not uniform. According to Rutherford's model most of the mass and positive charge of an atom is concentrated in a very small region called the nucleus.
The nucleus at the center of atom is surrounded by negatively charged particles which balance the positive charge on the nucleus. Thus the atom as a whole is electrically neutral.
Thus the negative charge is distributed through out the positive charge is the wrong hypothesis of Thomson's model.
To know more about Rutherford's model, visit;
https://brainly.com/question/30366944
#SPJ7
identify the acid associated with each conjugate base. nh3 choose... I⁻ ___
SO4²⁻ ___
Cl⁻ ___ OH⁻ ___
F⁻ ___
a. HF
b. Water
c. Sulfuric acid d. Hydronium ion e. HCI f. НІ g. Bisulfate ion
Answers
The acid associated with \(NH_3\) is \(NH_4^+\), with I- is HI, with \(SO_4^{2-}\) is \(HSO_4^-\), with Cl- is HCl, with OH- is \(H_2O\), and with F- is HF.
1. NH3: It is a base that accepts a hydrogen ion (H+) from an acid. \(NH_3 + H^+ --> NH_4^+\). The acid associated with \(NH_3\) is \(NH_4^+\).
2. I-: is a base that accepts a hydrogen ion (H+) from an acid. \(I^- + H^+ --> HI\) . The acid associated with I- is HI.
3. \(SO_4^{2-}\) : is a base that accepts a hydrogen ion (H+) from an acid. \(SO_4^{2-} + H^+ --> HSO_4^-\). The acid associated with \(SO_4^{2-}\) is \(HSO_4^-\).
4. Cl-: is a base that accepts a hydrogen ion (H+) from an acid. Its conjugate acid is the species formed when Cl- accepts a hydrogen ion (H+). \(Cl^- + H^+ --> HCl\). The acid associated with Cl- is HCl.
5. OH-: It is a base that accepts a hydrogen ion (H+) from an acid. Its conjugate acid is the species formed when OH- accepts a hydrogen ion (H+). \(OH^- + H^+ --> H_2O\). The acid associated with OH- is \(H_2O\).
6. F-: It is a base that accepts a hydrogen ion (H+) from an acid. \(F^- + H^+ --> HF\). The acid associated with F- is HF.
To learn more about acid click here https://brainly.com/question/29796621
#SPJ11
Aqueous sodium carbonate reacts with aqueous iron (II) chloride to produce solid iron (II) carbonate and aqueous sodium chloride with a yield of 92.5 %. Calculate the mass (in grams) of solid copper (II) carbonate produced if 94.7 mL of 0.121 M iron (II) chloride reacts with 2.56 x1021 formula units of sodium phosphate.
Answers
In the given chemical reaction 455 grams of solid iron (II) carbonate will be formed.
The balanced chemical equation for the reaction is:
Na2CO3 + FeCl2 ---> FeCO3 + 2NaCl
From the equation, we can see that the stoichiometry of Na2CO3 to FeCO3 is 1:1. So, the moles of FeCO3 produced will be the same as the moles of Na2CO3 used.
First, let's calculate the moles of FeCl2:
moles of FeCl2 = concentration x volume
moles of FeCl2 = 0.121 mol/L x 0.0947 L
moles of FeCl2 = 0.0115 mol
The reaction yield is 92.5%, so the actual moles of FeCO3 produced will be:
moles of FeCO3 = yield x moles of Na2CO3
moles of FeCO3 = 0.925 x (2.56 x 10^21 / Avogadro's number)
moles of FeCO3 = 0.925 x 4.25 mol
moles of FeCO3 = 3.93 mol
Therefore, the mass of FeCO3 produced can be calculated using its molar mass:
mass of FeCO3 = moles of FeCO3 x molar mass
mass of FeCO3 = 3.93 mol x 115.86 g/mol
mass of FeCO3 = 455 g
Therefore, it can be inferred that 455 grams of solid iron (II) carbonate will be produced.
Learn more about balanced chemical equation :
https://brainly.com/question/28294176
#SPJ4
The concentration of CO_2 in the Earth's atmosphere prior to significant human influences was
390ppm 280ppm 480ppm 160ppm
Answers
The Earth's atmospheric CO2 concentration was around 280ppm before human activities, but since the Industrial Revolution, burning fossil fuels has increased it to above 400ppm, well outside the range.
The concentration of CO2 in the Earth's atmosphere prior to significant human influences was 280ppm.
Before human activities, such as burning fossil fuels, deforestation, and industrial processes, the concentration of carbon dioxide in the atmosphere was relatively stable for thousands of years. This pre-industrial concentration of CO2 was around 280 parts per million (ppm).
To put it into perspective, 280ppm means that for every million molecules of air, around 280 of them were CO2 molecules. This level was maintained through a balance between natural sources of CO2, like volcanic activity and respiration, and natural sinks, such as photosynthesis and ocean absorption.
Since the Industrial Revolution, the burning of fossil fuels has significantly increased the concentration of CO2 in the atmosphere. Currently, the concentration is above 400ppm, which is considered to be well outside the range seen in the past 800,000 years.
In summary, the concentration of CO2 in the Earth's atmosphere prior to significant human influences was approximately 280ppm.
To know more about atmospheric Visit:
https://brainly.com/question/32274037
#SPJ11
what is the general form of the solubility product constant expression? how does the solubility quotient, qsp, differ? how do you calculate the value of qsp if given the solution concentration of two ions.
Answers
The general form of the solubility product constant (Ksp) expression is: Ksp = [A]^m[B]^n. By simply substituting the ion concentrations of the solution into the Ksp expression we can solve the Qsp.
The solubility quotient (Qsp) is similar to Ksp but represents the ion product in a solution, regardless of whether the solution is at equilibrium or not. If Qsp < Ksp, the solution is unsaturated and more solute can dissolve. If Qsp = Ksp, the solution is at equilibrium and the solution is saturated. If Qsp > Ksp, the solution is supersaturated and the excess solute will precipitate out of solution. To calculate Qsp, simply substitute the ion concentrations of the solution into the Ksp expression and solve for Qsp.
To know more about solubility quotient, here
brainly.com/question/28170449
#SPJ4