increasing ocean acidification has been attributed to . a. ocean absorption of excess atmospheric carbon dioxide. b. higher evaporation rates associated with climate change. c. changes in thermohaline circulation associated with the pacific decadal oscillation. d. freshwater inputs from melting ice.
Answers
Increasing ocean acidification has been attributed to. a. ocean absorption of excess atmospheric carbon dioxide.
In the field of biology, ocean acidification can be described as a process in which the acid quantity in the ocean increases due to an increase in the amount of carbon dioxide in the ocean.
When excess carbon dioxide is absorbed by the ocean from the atmosphere, then this carbon dioxide serves as a reacting agent for acidic compounds.
When the acid amounts in the ocean are increased then this can result in the death of living organisms in the oceans as they cannot survive in acidic environments.
To learn more about ocean, click here:
https://brainly.com/question/7604502
#SPJ4
Related Questions
Use LeChâtelier's principle explain why the concentration of NO at equilibrium increases when the reacuon takes place at higher temperatures
Answers
The increase in temperature causes the concentration of NO at equilibrium to increase because the reaction shifts to the left in order to relieve the stress caused by the increased temperature.
LeChâtelier's principle states that when a system at equilibrium is subjected to a stress, it will shift in a direction that tends to relieve that stress. In the case of the reaction of nitrogen monoxide (NO) with oxygen to form nitrogen dioxide (NO2), increasing the temperature is a stress that disturbs the equilibrium of the reaction.
At higher temperatures, the reaction will proceed more quickly due to the increased kinetic energy of the reacting species. This means that more NO2 will be formed, causing the concentration of NO2 to increase. According to LeChâtelier's principle, the reaction will shift to the left in order to relieve this stress and restore equilibrium. This means that the concentration of NO will increase, while the concentration of NO2 will decrease.
To know more about temperature
https://brainly.com/question/11464844
#SPJ4
URGENT!!-- Please help!
A mixture consists of 45% oxygen, 12% argon, and 43% nitrogen by volume. A sample of this mixture has a pressure of 2.0 atm in a 5.3 L container at 350 K temperature. How many moles of gas are in the container?
Answers
Moles of gas = 0.369
Further explanationGiven
P = 2 atm
V = 5.3 L
T = 350 L
Required
moles of gas
Solution
Ideal gas Law
\(\tt n=\dfrac{PV}{RT}\\\\n=\dfrac{2\times 5.3}{0.082\times 350}\\\\n=0.369\)
Avogadro's law : at the same temperature and pressure, the ratio of gas volume will be equal to the ratio of gas moles
moles of O₂ = 45% x 0.369 = 0.166
moles of Ar = 12% x 0.369 = 0.044
moles of N = 43% x 0.369 = 0.159
Which of the following could be the subshell representation of an element of Group IV?
A. s 2
B. s 2p 3
C. s 2p 2
D. s 2p l
Answers
The subshell representation of an element of Group IV is option C. s² p², since it contains 4 valence electrons.
What are Groups?In the Periodic Table, the Groups are the columns. For the Main-group elements, the group number coincides with the number of electrons in the valence shell.
Which of the following could be the subshell representation of an element of Group IV?
A. s². No, since there are 2 valence electrons.
B. s² p³. No, since there are 5 valence electrons.
C. s² p². Yes, since there are 4 valence electrons.
D. s² p¹. No, since there are 3 valence electrons.
The subshell representation of an element of Group IV is option C. s² p², since it contains 4 valence electrons.
Learn more about Groups here: https://brainly.com/question/24144303
#SPJ1
pls answer question will mark brainliset tyty

Answers
Answer:
A b/c aluminum
Explanation:
don't forget to follow rate like
Consider the following for both SN1 and SN2 reaction conditions:
◦ The nature of the leaving group (Cl vs. Br) in the 1-halobutanes
◦ The effect of the structure, i.e. compare:
◦◦◦◦ 1o, 2o, and 3o halides. Unhindered 1o vs. hindered 1o halides
◦◦◦◦ simple 3o vs. a comple 3o halide
◦◦◦◦ an allylic halide vs. a 3o halide
◦ The effect of solvent polarity on SN1 and SN2 reactions
◦ The effect of temperature on SN1 and SN2 reations
Answers
SN1 reactions are favored at higher temperatures.SN2 reactions are favored at lower temperatures
What is Nature of the leaving group?SN1 vs. SN2 reaction conditions:
Nature of the leaving group: SN1 reactions favor better leaving groups, such as Cl over Br. In SN2 reactions, the nature of the leaving group is less important.
Effect of structure:
1o, 2o, and 3o halides: SN1 reactions are favored for 3o halides due to carbocation stability. SN2 reactions are favored for 1o halides due to steric hindrance. 2o halides can undergo either SN1 or SN2 reactions depending on the specific conditions.
Hindered 1o vs. unhindered 1o halides: SN2 reactions are favored for unhindered 1o halides due to less steric hindrance. Hindered 1o halides may undergo either SN1 or SN2 reactions depending on the specific conditions.
Simple 3o vs. complex 3o halides: SN1 reactions are favored for simple 3o halides due to carbocation stability. Complex 3o halides may undergo either SN1 or SN2 reactions depending on the specific conditions.
Allylic halide vs. 3o halide: Allylic halides may undergo SN1 or SN2 reactions depending on the specific conditions. 3o halides generally undergo SN1 reactions due to carbocation stability.
Effect of solvent polarity: SN1 reactions are favored in polar solvents that stabilize the carbocation intermediate, while SN2 reactions are favored in aprotic solvents that solvate the nucleophile and prevent ion pairing with the substrate.
Effect of temperature: SN1 reactions are favored at higher temperatures due to the increased energy required to form the carbocation intermediate. SN2 reactions are favored at lower temperatures due to the decreased energy required for the nucleophile to approach the substrate.
Learn more about SN1 reactions & SN2 reactions
brainly.com/question/27548297
#SPJ11
7. Define the terms filtrate, mother liquor, tare weight, solute.
Answers
Filtrate refers to the liquid that passes through a filter during the process of filtration. Mother liquor is the concentrated liquid that remains after a substance has been crystallized or precipitated out of a solution. Tare weight is the weight of an empty container or vessel that is used to hold a substance for weighing. Solute is a substance that is dissolved in a solvent to form a solution.
1. Filtrate: Filtrate refers to the liquid that has passed through a filter, leaving behind any solid particles or impurities. In this process, the filter separates the liquid from the solid components of a mixture.
2. Mother liquor: Mother liquor is the remaining liquid after a solute has been crystallized and removed from a solution. It typically contains dissolved impurities and some uncrystallized solute that did not separate during the crystallization process.
3. Tare weight: Tare weight is the weight of an empty container or vehicle. It is typically used in weighing systems to account for the container's weight so that only the weight of its contents (i.e., the net weight) is measured.
4. Solute: A solute is a substance that is dissolved in a solvent to form a solution. It is the component of the solution that is present in a lesser amount, and its particles are uniformly distributed throughout the solvent.
To know more about Mother Liquor:
https://brainly.com/question/30666181
#SPJ11
Nuclear fission occurs when _______________ a. TNT and plutonium are combined, causing the molecules to separate. b. a nucleus breaks up into two equal fragments that release and separate more atoms. c. like atoms collide to create double nuclei. d. trinitite is created by multiple molecules that form a single atom.
Answers
Nuclear fission occurs when a nucleus breaks up into two equal fragments that release and separate more atoms. So, the correct option is B.
Nuclear fission is a process in which the nucleus of an atom breaks apart into two or more smaller nuclei. This process releases a significant amount of energy.
Option B accurately describes the process of nuclear fission. When a heavy nucleus, such as uranium-235 or plutonium-239, absorbs a neutron, it becomes unstable and splits into two smaller nuclei.These smaller nuclei, along with additional neutrons, are released in the process. The release of neutrons can trigger a chain reaction, where each neutron released can potentially collide with other nuclei, causing them to undergo fission as well.The energy released during nuclear fission is due to the conversion of a small amount of mass into a large amount of energy, as described by Einstein's famous equation, E=mc².This energy is utilized in various applications, including nuclear power generation and nuclear weapons. Nuclear fission reactions are carefully controlled in nuclear power plants to ensure the sustained release of energy without leading to uncontrolled chain reactions. Hence the correct option is B.
For more questions on nucleus
https://brainly.com/question/29855834
#SPJ8
Between reaction of magnesium powder and lead oxide which is oxidized in the reaction and which is oxidizing agent
Answers
Answer:
Mg is oxidized and Pb is the oxidizing agent
Explanation:
Determine whether each of the following atoms will form a nonpolar covalent compound or a polar covalent compound, and give the formula of the compound.
(a) 2 oxygen
(b) hydrogen and bromine
(c) oxygen and 2 hydrogen
(d) 2 iodine
Answers
(a) Nonpolar covalent compound: O2 , (b) Polar covalent compound: HBr, (c) Nonpolar covalent compound: H2O and (d) Nonpolar covalent compound: I2 .
What is Nonpolar ?Nonpolar molecules are molecules that have no electrical charge and a symmetrical distribution of electrons. This means that the molecules have a uniform distribution of electrons around its nucleus, creating no electrical imbalance. Nonpolar molecules are not attracted to other molecules, so they tend to be more stable and have lower boiling points than molecules with electrical charges. Examples of nonpolar molecules include methane, carbon dioxide, and hydrogen. Nonpolar molecules are also hydrophobic, meaning they do not mix with water, so they tend to be less soluble in water than polar molecules.
To learn more about Nonpolar
https://brainly.com/question/1426521
#SPJ4
in the photosynthesis reaction, 6co2 + 6h2o → c6h12o6 + 6o2, there are 18 oxygen atoms in the reactants. how many oxygen atoms are in the products?
Answers
There are also 6 oxygen atoms in the products. This is because the products of the photosynthesis reaction include C6H12O6 (glucose) and 6O2 (oxygen gas). The glucose molecule contains 6 carbon, 12 hydrogen, and 6 oxygen atoms.
In the photosynthesis reaction, 6CO2 + 6H2O → C6H12O6 + 6O2, there are 18 oxygen atoms in the reactants.
Therefore, the total number of oxygen atoms in the products is 6. This is because oxygen gas is not bonded to anything else and is released into the atmosphere as a product of photosynthesis.
The reactants of photosynthesis, carbon dioxide (CO2) and water (H2O), are converted into glucose (C6H12O6) and oxygen (O2) gas. In this process, sunlight energy is converted into chemical energy that is stored in glucose, which is used by the plant for growth and other metabolic processes. Therefore, photosynthesis is a crucial process for life on earth.
To know more about photosynthesis visit:
https://brainly.com/question/30715543
#SPJ11
1. How many mL of copper (II) sulfate of a 2.5M stock solution is needed to make 250 mL of 0.2M solution?
Answers
Answer:
20ml
Explanation:
using the formula;C1V1=C2V2
2.5×V1=0.2×250
2.5V1= 50
V1=50/2.5
V1=20L
what is the bond order of the n-o bonds in the nitrosyl fluoride molecule?
Answers
The bond order of the N-O bonds in nitrosyl fluoride (NOF) is 1.5.
Bond order refers to the number of chemical bonds present between two atoms in a molecule. It is calculated based on the number of electrons involved in bonding and can range from zero to a maximum of 3 for a covalent bond.
In nitrosyl fluoride (NOF), the nitrogen (N) atom and the oxygen (O) atom are joined by a single covalent bond, which involves the sharing of two electrons. However, the bond is not a simple single bond, but rather a resonance hybrid of two contributing structures, both of which have a bond order of 1.5. This results in a bond order of 1.5 for the N-O bond in nitrosyl fluoride.
The concept of bond order is important because it provides insight into the stability of a molecule and helps to predict the bond lengths and strengths of the bonds in the molecule.
Learn more about bond order here:
https://brainly.com/question/2705237
#SPJ4
a fractional distillation involves the use of a fractionating column to provide multiple condensation/evaporation cycles over a given distance. group of answer choices true false
Answers
The given statement "A fractional distillation that involves the use of the fractionating column and to provide the multiple condensation or the evaporation cycles over the given distance" is true as it involves the separation of the miscible liquids.
The Fractional distillation is the type of the distillation that will involves the separation of the miscible liquids. This process will involves the repeated distillations and the condensations. The mixture is separated into the component parts. The separation that happens when the mixture will be heated at the certain temperature and the fractions of the mixture will start to vaporize.
The more will be the volatile components will increase in the vapor state after the heating, and when it is liquefied, the volatile components increase in the liquid state.
To learn more about Fractional distillation here
https://brainly.com/question/27004370
#SPJ4
Ocean tides are caused by the___ attraction of the moon and sun.
Please help me. Asap
Answers
Answer:
Gravitational
Explanation:
please mark brainliest
A sample of gas has an initial volume of 15 L and an initial pressure of 4.5 atm. If the pressure changes to 1.8 atm, what is the new volume, assuming that the temperature remains constant?
Answers
Answer:
37.5 L
Explanation:
Initial Volume, V1 = 15L
Initial Pressure P1 = 4.5 atm
Final Pressure, P2 = 1.8 atm
Final Volume V2 = ?
The relationship between these variables is given as;
P1V1 = P2V2
V2 = PIV1 / P1
Inserting the values;
V2 = 4.5 * 15 / 1.8
V2 = 37.5 L
Answer: The new volume is 37.5L
Explanation:
The question is solved using the principle and formula of a gas law known as the Boyle's law. Boyle's law states that provided the temperature remains constant, the volume of a given mass of gas is inversely proportional to its pressure. Expressing the statement mathematically:
P ∝ 1/V, (where p is equal to pressure and V is volume)
Therefore PV = a constant
If a gas at pressure P1 and Volume V1 changes at constant temperature to pressure P2 and volume V2 then:
P1V1 = P2V2.
Using the formula to solve the question:
P1 = 4.5atm
P2 = 1.8atm
V1= 15L
V2 = ?
V2 = P1V1/P2
V2= 4.5 × 15 /1.8
V2 = 67.5/1.8
V2 = 37.5 L
Therefore the new volume is 37.5L
How does the amount of baking soda used affect the size of the cookie baked?
Answers
The table above summarizes data given to a student to evaluate the type of change that took place when substance X was mixed with water. The student claimed that the data did not provide enough evidence
to determine whether a chemical or physical change took place and that additional tests were needed. Which of the following identifies the best way to gather evidence to support the type of change that
occurred when water and X were mixed?
Answers
The question is incomplete, the complete question is;
The table above summarizes data given to a student to evaluate the type of change that took place when substance X was mixed with water. The student claimed that the data did not provide enough evidence to determine whether a chemical or physical change took place and that additional tests were needed. Which of the following identifies the best way to gather evidence to support the type of change that occurred when water and Xwere mixed?
A. Measuring the melting point of the mixture of water and X
B. Adding another substance to the mixture of water and X to see whether a solid forms
C Measuring and comparing the masses of the water, X, and the mixture of water and X
D Measuring the electrical conductivities of X and the mixture of water and X
Answer:
D Measuring the electrical conductivities of X and the mixture of water and X
Explanation:
Unfortunately, I am unable to reproduce the table here. However, from the table, the temperature of the of the mixture of the solid X and water was 101.6°C. This is above the boiling point of water and way below the temperature of the solid X.
This goes a long way to suggest that there was some kind of interaction between the water and X which accounted for the observed temperature of the system of X in water.
The only way we can be able to confirm if X actually dissolved in water is to measure the conductivity of the water. dissolved solids increase the conductivity of water.
The statement which identifies the best way to gather evidence to support the type of change that occurred when water and X were mixed is:
D Measuring the electrical conductivities of X and the mixture of water and XAccording to the given question, we are asked to show the statement which identifies the best way to gather evidence to support the type of change that occurred when water and X were mixed. \
As a result of this, we can see that from the complete question, there is a need to note the temperature of the solution which is 101.6°C and then to measure the conductivity of the water to know for sure the type of change that occured.
Therefore, the correct answer is option D
Read more here:
https://brainly.com/question/18384031
Question 2 of 10
How do collisions between molecules transfer energy from a system of
reacting substances to its surroundings?
OA. They cause heat to flow from the system to the surroundings.
OB. They cause the thermal energy of the surroundings to decrease.
OC. They cause the potential energy of the system to increase.
OD. They cause molecules to flow into the system from the
surroundings.
SUBMIT
Answers
The correct answer is "They cause heat to flow from the system to the surroundings." (Option A)
What evidence is there in your experiment that the water collected in the receiving flask was salt free?
Answers
There are numerous methods for determining if the water collected in the receiving flask during the experiment is salt-free or not like taste test, conductivity test and chemical analysis.
To establish this, the experimenter may utilise one or a combination of the approaches listed below:
Taste Test: Tasting the water is the simplest way to check if it is salt-free. If there is no discernible salt flavour in the water, it is most likely salt-free.Conductivity Test: The electrical conductivity of water is increased by the presence of salts in it. The experimenter can detect if the water is salt-free by measuring the electrical conductivity of the water in the receiving flask.Chemical Analysis: The experimenter may choose to do a chemical analysis on the water to identify the presence of salts. This can be accomplished using techniques such as ion chromatography or atomic absorption spectroscopy.For more such questions experiment.
https://brainly.com/question/11256472#
#SPJ11
There are three stable atoms of Argon (Atomic Number 18): Argon-36, Argon-38 and
Argon-40. What would the atoms of these isotopes have in common? What would
be different about their atoms? (4 points)
HELP ASAP
Answers
On the periodic table, argon has an average atomic weight of 39.948 amu. This number is really near 40. This suggests that Ar-40 is the isotope of argon (Ar) that is most prevalent in the natural world.
What characteristics do argon-36, argon-38, and argon-40 share?If all three argon atoms are neutral, they would each contain 18 protons and 18 electrons. In comparison to one another, the three isotopes will each have a distinct number of neutrons (18, 20, and 22 neutrons respectively).
Why is potassium a 39 positioned before argon, atomic number 40, in the current periodic table?Atomic number, not atomic mass, is used to categorise the elements. As the atomic number of argon (18) is less than that of potassium (19)
To know more about periodic table visit:-
brainly.com/question/11155928
#SPJ1
A gas with a volume of 250 mL at a temperature of 293K is heated to 324K. What is the new volume of the gas?
Answers
Answer:
you can simply answer vl/t1=v2/t2
Typhoons are large storms that form over the Pacific Ocean Hurricanes are large storms that form over the Atlantic Ocean. Which of the following are essential components in the formation of a hurricane
or a lyphoon
A High pressure, high temperature, cyclonic winds, dry air
B. High pressure cool temperature, strong winds, humid air
cWarm front, clouds, low pressure, high slevation
D Low pressure, warm temperature, warm ocean waters, spiraling winds

Answers
My father rode out a typhoon near Okinawa WWII, onboard the battleship USS Missouri BB-63.
Violent pitching, alarms going off for approaching capsize pitch. The captain came on loudspeaker “ don’t worry men, land is near... about a mile straight down”.
hello can anyone help thx
Potassium iodide is an ionic compound.
Describe what happens, in terms of electron loss and gain, when a potassium atom reacts
with an iodine atom.
Answers
Answer:
In this reaction, potassium atoms lose electrons to form positively-charged potassium ions (oxidation) and iodine atoms gain electrons to form negatively-charged iodide ions (reduction).
Explanation:
pls mark as brainliest
In this process, iodine atoms get electrons to produce negatively charged iodide ions, whereas potassium atoms lose electrons to form positively charged potassium ions. Oxidation and reduction takes place.
What is oxidation ?Redox reactions include a change in the oxidation state of the substrate. Similar to how reduction is the acquisition of electrons while oxidation is the loss of electrons or an increase in the oxidation state.
An oxidation process takes place when oxygen interacts with a substance or an element. Another way to think of oxidation is as the process of removing hydrogen from the species of reactants. Molecule, atom, or ion oxidation is the process of losing electrons.
Because they generate new functional groups in molecules or alter existing functional groups, oxidation processes are crucial in the synthesis of organic substances.
Thus, the iodine atom also experiences a change. The iodide atom gets one electron during the reaction with potassium, leaving it with eight electrons at its outer energy level.
To learn more about oxidation, follow the link;
https://brainly.com/question/13182308
#SPJ2
how can i determine salt base and acid
Answers
Answer: You can do this by using ph strips. It should determine which is a salt base and an acid!
How many carbon atoms are in 1.25 mol of silver acetate?
Answers
The number of carbon atoms present in 1.25 mol of silver acetate is 1.875 × 10²³.
What is Avogadro's number?Avogadro's number is define as the number of atoms of any substance present in one mole of that substance, and it is equal to 6.022 × 10²³, i.e.
1 mole = 6.022 × 10²³ atoms/mol
Given moles of silver acetate = 1.25 moles
Number of atoms of silver acetate in 1.25 moles = 1.25 × 6.022 × 10²³ = 7.5×10²³
Molecular formula of silver acetate is CH₃CO₂Ag, means in this molecule total 8 atoms are present so number of carbon atom will be calculated as:
Carbon atoms = (2× 7.5 × 10²³) / 8 = 1.875 × 10²³ atoms [here we multiply by 2 because 2 atoms of carbon is present in olecule]
Hence required atoms of carbon is 1.875 × 10²³.
To know more about Avogadro's number, visit the below link:
https://brainly.com/question/1513182
12. State duplet and duplet rule. Explain in brief with examples .
Answers
Answer:
I HOPE THE ABOVE INFORMATION WILL HELP YOU A LOT.

What type of reaction does this illustration represent?
A. synthesis
B. decomposition
C.single-replacement
D.double replacement

Answers
Answer: IS B
Explanation:
Decomposition
h
please help me with my question I will like and mark as brainliest NO LINKS THEY DON'T WORK AND IF U DON'T KNOW THE ANSWER PLS DON'T ANSWER AT ALL

Answers
Answer:
Hehehe :)
Interesting.
Phase 1.
Zinc heated in Air
2Zn + O₂ = 2ZnO
Zinc Oxide is formed.
SOLID A = (ZnO)ZINC OXIDE
Phase 2.
Zinc Oxide is heated with Powdered Magnesium.
magnesium would displace Zinc since its higher in the series.
ZnO + Mg = MgO + Zn
SOLID B + SOLID C = MgO(Magnesium Oxide) + Zn(Zinc metal)
At Phase 3...( 1st Aspect)
Both MgO and Zn can react with Dilute HCl.
Now the products required from the question are a "SOLUTION D" AND "GAS E".
When MgO reacts with Dilute HCl
The products are
MgO + 2HCl = MgCl₂ + H₂O
Now H₂O could be steam(gas) or Water :(
This is a little Uncertain.
But if we use Zn as the one reacting with Dilute HCl
We have
Zn + 2HCl = ZnCl₂ + H₂
Now I'll go with this cos H₂ is simply Hydrogen Gas.
SOLUTION D + GAS E = ZnCl₂(Zinc Chloride) + H₂(Hydrogen Gas)
2nd aspect of Phase 3 ( or denominator)
ELECTROLYSIS OF MOLTEN B
B = MgO
MOLTEN B = MOLTEN MAGNESIUM CHLORIDE.
From Electrolysis of MgO
Mg²⁺ + 2e- = Mg
2O²⁻ = O₂ + 2e⁻
Combining Both Equations.
Mg²⁺ + 2O²⁻ = 2Mg(ₛ) + O₂(g)
Magnesium is a Silvery Metal :)
and Oxygen is a Gas.
Wow!!!
So this automatically makes "SILVERY METAL F" AND "GAS G" TO BE MAGNESIUM AND OXYGEN RESPECTIVELY.
SILVERY METAL F = MAGNESIUM
SILVERY METAL F = MAGNESIUMGAS G = OXYGEN
All the way down to phase 5 now.
Hope you're with me?
Now CuSO₄ is added to Mixture of Mg and O₂. Oxygen would prolly escape into the atmosphere
Leaving Mg to react with CuSO₄
Mg + CuSO₄ = MgSO₄(aq) + Cu(s)
Copper when precipitated from solution sometimes has Brown Pink Color.
MgSO₄ is Solid.
Niceeeee
That's exactly what they need as the answer.
Automatically...
BROWN PINK SOLID H = COPPER(CU)
SOLUTION I = MgSO₄(MAGNESIUM SULPHATE)
Hope this Helps :)
Have a great day.
what is 15 /2.343*233+2341
if you are reading this you like the person you are sitting beside or just beside or will sit beside next.
Answers
Answer:
3832.67
Explanation:
I need help on this please
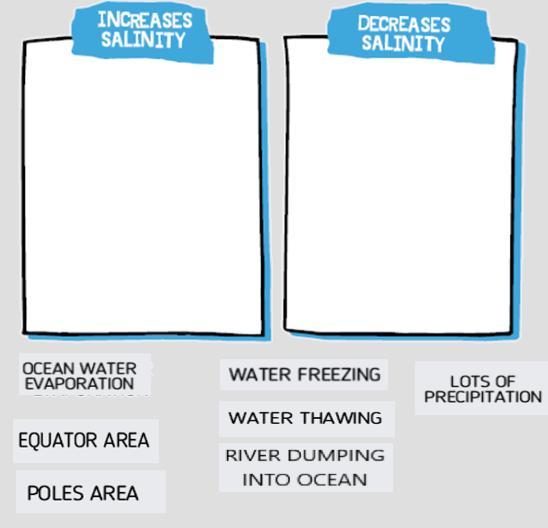
Answers
Lots of precipitation, ocean water evaporation and water freezing increases salinity. Equator area, poles area, water thawing and river dumping into ocean decreases salinity.
What is salinity?Based on its saltiness, salted ice forms at a certain temperature. More salty water takes longer to freeze than less saline water does. The boiling point of a salty solution is greater than that of fresh water. Due to its impact on salinity, evaporation is also lower over more salinized water than over less salinized water. Saline seawater has a higher density than freshwater.
The overwhelming bulk of oceanic salinity is found in coastal seas. Salts are brought into solution by rivers from continental regions. It's interesting to note how differently sea salt as well as freshwater salt are made. Lots of precipitation, ocean water evaporation and water freezing increases salinity. Equator area, poles area, water thawing and river dumping into ocean decreases salinity.
Therefore, lots of precipitation, ocean water evaporation and water freezing increases salinity.
To learn more about salinity, here:
https://brainly.com/question/14575146
#SPJ1