in the procedure why do we need to dissolve the iodine in methanol
Answers
In the procedure, we need to dissolve iodine in methanol to allow the iodine to dissolve and be extracted. This allows us to analyze the solution and measure the concentration of iodine in the solution.
We need to dissolve the iodine in methanol because it is a good solvent for iodine. Iodine is relatively insoluble in water, but it dissolves well in methanol. By dissolving the iodine in methanol, we can create a homogenous solution that can be used for various purposes, such as titrations or other analytical procedures. Methanol also has a relatively low boiling point, which means it can be easily removed from the solution if needed. Overall, the use of iodine in methanol is an important step in many chemical procedures.
Here you can learn more about iodine https://brainly.com/question/16867213
#SPJ11
Related Questions
Luke's collection has 27 postcards in it than brian's collection. Put togteher, their collections have 135 postcards how many postcards does luke have how many postcards does brian have
Answers
Answer:
I. Luke has 81 postcards.
II. Brian has 54 postcards.
Explanation:
Let Luke's collection be L
Let Brian's collection be B
Translating the word problem into an algebraic equation, we have;
L = 27 + B
L + B = 135
Substituting equation 1 into equation 2, we have;
27 + B + B = 135
27 + 2B = 135
2B = 135 - 27
2B = 108
B = 108/2
B = 54
Therefore, Brian has 54 postcards.
To find the value of L;
L = 27 + B
L = 27 + 54
L = 81
Therefore, Luke has 81 postcards
early atomic theories could not explain the ______ of elements
Answers
Explanation:
But I think properties of the element
because at past people were not well informed about the properties of element so later modern periodic table replaces with mendellive periodic table correcting it's bud and mistakes.
Rank the substances involved in this reaction in the order of decreasing potential energy:
Mg(oh)2(s) + CO2(g) ⇄ MgCO3(s) + H2O(g)
Answers
The answer is- The order of decreasing potential energy is :
\(CO_{2}(g) > Mg(OH)_{2} (s) > MgCO_{3} (s)\)
The potential energy can be expressed as the static energy that is stored in the chemical bonds.
What is the trend of potential energy in an exothermic reaction and an endothermic reaction?
During an exothermic reaction, heat is released and the stored potential energy of bonds is converted into the kinetic energy and thus there is a decrease in the potential energy during an exothermic reaction.During an endothermic reaction, heat is absorbed during the reaction and this heat is stored as potential energy in bond, hence the potential energy increases during a endothermic reaction.Now, during this reaction, basic Magnesium hydroxide, \(Mg(OH)_{2}\)reacts with acidic \(CO_{2}\), thus this reaction is an exothermic reaction.Hence, the potential energy of products is less than the potential of reactants.Next, the potential energy of solids are always more than the potential energy of gases.Therefore, the decreasing order of potential energy is\(CO_{2}(g) > Mg(OH)_{2} (s) > MgCO_{3} (s)\) [H2O is not considered]
To learn more about the potential energy, visit:
https://brainly.com/question/19569739
#SPJ4
someone help me on these two 2

Answers
Answer:
Question 4 is- Solubility
Question 5 is- Suspension
Hopes this helps >:D
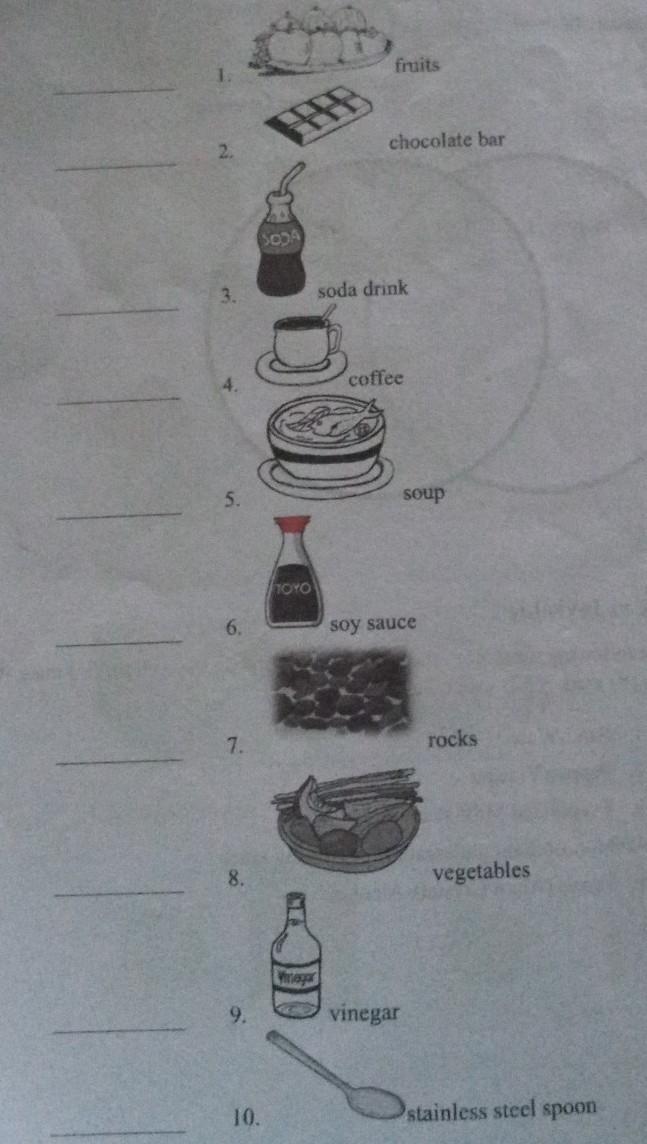
uch Activity 2 - Look at Me Directions: Given the illustrations below, tell whether it is a homogeneous mixture or heterogeneous mixture. Write HM on the blank before each item if it is homogeneous or HT if it is heterogeneous.
Answers
Answer:
my family will visit our grandparents
Answer:
my family also visit thier grandmother's
a student proposes the following lewis structure for the peroxide ion. assign a formal charge to each atom in the student's lewis structure.
Answers
A student proposes the following lewis structure for the peroxide ion.
what is formal charge?
A hypothetical charge that might be present on an atom in a molecule is known as a formal charge. It makes the corresponding polarity assumption that sharing electrons are equally spaced from the parent atoms. Polarity is the presumption that, unless parent atoms are identical, such as H-H, electrons are never equally distant from them.
For a polyatomic ion, the formal charges must add up to the ion's charge rather than the sum of the formal charges on all the atoms in the molecule (which may be positive or negative). Formal Charge = [Valence electron count in a single atom] - [(number of bonding electrons) 12 (number of lone pair electrons)]
The peroxide has a formal charge of -2.
To learn more about formal charge from the given link below,
https://brainly.com/question/13565135
#SPJ4
according to lewis dot theory, what types of electron pairs would you find surrounding the central atom of the nitrite ion?
Answers
According to Lewis dot theory, you would find one lone pair and two bonding pairs of electrons surrounding the central atom of the nitrite ion.
The nitrite ion (NO2-) is composed of a nitrogen atom bonded to two oxygen atoms. According to Lewis dot theory, the central atom (nitrogen) is surrounded by a total of three electron pairs. Two of these electron pairs are involved in covalent bonds with the oxygen atoms, while the third electron pair is a lone pair that resides on the nitrogen atom.
The two bonding pairs of electrons between the nitrogen and oxygen atoms are represented by a single line in the Lewis structure, indicating a single covalent bond. The lone pair of electrons on the nitrogen atom is represented by two dots, or a pair of dots, indicating that these electrons are not involved in bonding.
Overall, the Lewis dot structure for the nitrite ion shows one lone pair and two bonding pairs of electrons surrounding the central atom (nitrogen). This arrangement of electron pairs is important for understanding the shape and polarity of the molecule, as well as its reactivity and chemical properties.
To know more about the Lewis dot, here
https://brainly.com/question/20300458
#SPJ4
if i want to make 14 moles of potassium chloride, how many grams of potassium do i need? Show all work. Make sure to include unit and substance

Answers
Answer:
The answer is 0.013413582325191. i assume you are converting between moles Potassium Chloride and gram. The molecular formula for Potassium Chloride is KCl. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Potassium Chloride, or 74.5513 grams.
Explanation:
complete and balance the following oxidation–reduction reaction in basic solution: cr(oh)3(s) clo−(aq)−→−cro42−(aq) cl2(g)cr(oh)3(s) clo−(aq)→ cro42−(aq) cl2(g) When this equation is balanced using the smallest whole-number coefficients, what is the coefficient on H2O, and on which side of the reaction is H2O found, product side or reactant side?(a) 2, reactant side(b) 8, product side(c) 12, reactant side(d) 2, product side(e) 14, product side
Answers
The balanced equation for the oxidation–reduction reaction in basic solution is:
3Cr(OH)3(s) + 4ClO−(aq) → 3CrO42−(aq) + 2Cl2(g) + 6OH−(aq)
The coefficient on H2O is 0 because there is no water involved in this reaction. Therefore, the answer is not provided in the options.
To balance the given oxidation-reduction reaction in basic solution: Cr(OH)3(s) + ClO^-(aq) → CrO4^2-(aq) + Cl2(g), we follow these steps:
1. Assign oxidation numbers: Cr in Cr(OH)3 has a +3 oxidation state, and in CrO4^2-, it has a +6 oxidation state. Cl in ClO^- has a +1 oxidation state, and in Cl2, it has a 0 oxidation state.
2. Balance the atoms that undergo oxidation and reduction:
2Cr(OH)3(s) → 2CrO4^2-(aq) (balance Cr)
3ClO^-(aq) → 3/2Cl2(g) (balance Cl)
3. Balance the charges by adding electrons:
2Cr(OH)3(s) + 6e^- → 2CrO4^2-(aq)
3ClO^-(aq) + 6e^- → 3/2Cl2(g)
4. Combine the two half-reactions:
2Cr(OH)3(s) + 3ClO^-(aq) → 2CrO4^2-(aq) + 3/2Cl2(g)
5. Balance the remaining atoms (O and H) using H2O and OH^- (as we are in basic solution):
2Cr(OH)3(s) + 3ClO^-(aq) + 6OH^-(aq) → 2CrO4^2-(aq) + 3/2Cl2(g) + 6H2O(l)
Multiplying the entire equation by 2 to remove the fraction:
4Cr(OH)3(s) + 6ClO^-(aq) + 12OH^-(aq) → 4CrO4^2-(aq) + 3Cl2(g) + 12H2O(l)
The smallest whole-number coefficient for H2O is 12, and H2O is found on the product side of the reaction. Thus, the correct answer is (b) 8, product side.
Visit here to learn more about oxidation-reduction reaction : https://brainly.com/question/19528268
#SPJ11
Violet light has a wavelength of 4.10 x 10-12 m. What is the frequency?
Answers
Answer:
frequency =7.32 x 1019 hertz
To find the frequency you must time speed of light (3 x 10^8) times wavelength
Any material that exerts magnetic force is considered a magnet true or false?
Answers
Answer:
Any material that exerts magnetic force is considered a magnet.
TRUE
Green plants use light from the Sun to drive photosynthesis, a chemical reaction in which liquid water and carbon dioxide gas form aqueous glucose (C6H1206) and oxygen (O2) gas. Calculate the moles of water needed to produce 0.070 mol of oxygen. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.
Answers
0.070 moles of water are needed to produce 0.070 mol of oxygen.
To calculate the moles of water needed to produce 0.070 mol of oxygen, we should first determine the balanced equation for photosynthesis. The balanced equation is:-
6CO₂ + 6H₂O → C₆H₁₂O₆ + 6O₂
From this equation, we can see that 6 moles of water are required to produce 6 moles of oxygen. Therefore, we can set up a proportion to find the moles of water needed for 0.070 mol of oxygen:-
(6 moles H₂O / 6 moles O₂) = (x moles H₂O / 0.070 moles O₂)
Now, we can solve for x:-
x moles H₂O = (6 moles H₂O / 6 moles O₂) * 0.070 moles O₂
x moles H₂O = (1) * 0.070 moles O₂
x moles H₂O = 0.070 moles
Therefore, 0.070 moles of water are needed to produce 0.070 mol of oxygen.
Learn more about moles: https://brainly.com/question/29367909
#SPJ11
describe in your own words the structure of solid sodium chloride and explain why it is formula is NACL
Answers
Answer:
Sodium chloride is formed when sodium atoms interact with chlorine atoms. When this occurs, sodium will donate an electron (which is a negatively-charged particle) to chlorine. This makes sodium slightly positive and chlorine slightly negative.
Opposite charges attract, right? So then, sodium ions will attract chloride ions and form an ionic bond. By the way, chloride is the term used to designate the anion form of chlorine. The result is a crystallized salt that has properties that are different from the two parent elements (sodium and chlorine). The chemical formula for sodium chloride is NaCl, which means that for every sodium atom present, there is exactly one chloride atom.
An object has a mass of 183.5 g and a density of 14.8 g/cm³. Determine the volume of the objectin cm³.
Answers
First, let's remember the formula to calculate an object's density:
\(\begin{gathered} \rho=\text{ }\frac{m}{V} \\ \\ Being\text{ }\rho\text{ the density, m the mass, and V the volume.} \end{gathered}\)Then, we analyze what we have:
\(\begin{gathered} m\text{ = 183.5 g} \\ \rho=\text{ 14.8 g/cm}^3 \end{gathered}\)We need to determine the volume, so we transform our formula like this:
\(V=\text{ }\frac{m}{\rho}\)We replace our data:
\(V=\text{ }\frac{183.5\text{ g}}{14.8\text{ g/cm}^3}=\text{ 12.399 cm}^3\approx\text{ 12.4 cm}^3\)Then, the answer is that the volume equals 12.4 cm^3.
A solution made by dissolving licl in water to make 85. 0 g solution. The solution has a density of 1. 46 g/ml. The resulting concentration is 1. 60 m. How much licl is in the solution?.
Answers
There are 3.95 grams of \(LiCl\) in the solution.
The density of the solution is 1.46 g/mL, so the volume of the solution is:
volume = mass / density
volume = 85.0 g / 1.46 g/mL
volume = 58.22 mL
The concentration of the solution is 1.60 M, which means there are 1.60 moles of \(LiCl\) in 1 liter of solution. To find the number of moles of \(LiCl\)in the 58.22 mL of solution, we can use the following equation:
moles = concentration x volume (in liters)
First, we need to convert the volume of the solution to liters:
volume = 58.22 mL / 1000 mL/L
volume = 0.05822 L
Now we can calculate the number of moles of \(LiCl\) in the solution:
moles = 1.60 M x 0.05822 L
moles = 0.0932 moles
Finally, we can calculate the mass of\(LiCl\)in the solution using its molar mass:
mass = moles x molar mass
mass = 0.0932 moles x 42.39 g/mol
mass = 3.95 g
Therefore, there are 3.95 grams of \(LiCl\) in the solution.
To know more about molar mass refer to-
https://brainly.com/question/22997914
#SPJ11
Express the diameter of a ground-state hydrogen atom.
Answers
The diameter of a ground-state hydrogen atom can be calculated using the Bohr radius (a0) which is approximately equal to 0.529 Å (angstroms) and the formula for the diameter of a sphere (2 × radius).
So for the ground-state hydrogen atom (n = 1), the radius (r) would be :
\(r = a0 / 1\)
= a0 The diameter of a sphere is 2 times the radius so the diameter of a ground-state hydrogen atom can be expressed as follows:
Diameter = 2 × radius
Diameter = 2 × a0
Diameter = 2 × 0.529.
Diameter = 1.058 Å
Therefore, the diameter of a ground-state hydrogen atom is approximately 1.058 angstroms. This process has significant applications in the food and beverage industry as well as in the production of biofuels.
To know more about hydrogen visit:
https://brainly.com/question/30623765
#SPJ11
ignoring the effects of recoil, what minimum energy must the photon have for this reaction to occur? the mass of a 2814si atom is 27.976927 u , and the mass of a 2412mg atom is 23.985042 u .
Answers
Ignoring the effects of recoil, the minimum energy must the photon have for this reaction to occur is 10 MeV
The nuclear reaction is (28,14)Si+ γ---(24,12)Si+He(2,4)
The binding energy that holds the nuclide is
E=Δmc^2
where Δm is the mass defect
Δm=27.976927+0-[23.985042+4.002602]
Δm=-0.010717x1.66054x10^-27 kg
So the minimum energy of photon should be
ΔE=Δmc^2=0.017796x10^-27x(3x10^8)^2/1.6x10^-19 V
ΔE= 10 MeV
Binding energy is the energy required to separate a particle from a system of particles.The mass of any stable nucleus is found to be less than the sum of the mass of all the protons and neutrons inside the nucleus. This lost mass is stored in the form of energy that keeps the nucleus together and is termed as binding energy.To learn more about binding energy visit:
brainly.com/question/10095561
#SPJ4
Type the correct answer in each box.
Balance the chemical equation.
N₂O3-
N₂ +
0₂
Answers
The Balanced Reaction is 2N₂O₃⁻ -----> 2 N₂ + 3 0₂
What is a Balanced Reaction ?A reaction in which the number of atoms of each element in the reactant and the product are equal is called a Balanced Reaction .
It is given in the question:
To Balance the chemical equation.
N₂O₃⁻ -----> N₂ + 0₂
To balance the reaction , the number of molecules of each element , i.e. Nitrogen (N) and Oxygen (O) needs to be balanced
2N₂O₃⁻ -----> 2 N₂ + 3 0₂
To know more about Balanced Reaction
https://brainly.com/question/14280002
#SPJ1
Two paths in a park intersect so that one of the angles at the intersection is 75°. What are the three other angle measurements formed by the intersection? A. 15°, 75°, 175° B. 65°, 105°, 135° C. 75°, 105°, 105° D. 75°, 115°, 115°
Answers
To find the other angles, we can subtract 75° from 180° to get 105°. Then, since there are two other angles, we divide 105° by 2 to get 52.5°.
So that means, the three other angle measurements formed by the intersection are:
B. 65°, 105°, 135°
An ameba absorbs oxygen from its environment and releases carbon dioxide into its environment. This process is known as
Answers
Answer:
Respiratory gas exchange
Explanation:
What is the benefit to having baby steps in science occur as small bits of evidence accumulate?
If someone could help me I would really appreciate it! Thank you

Answers
Answer:
aby steps in science occur as small bits of evidence gradually accumulate. The accumulating evidence lets scientists refine and expand on earlier ideas
Explanation:
The benefit to having baby steps in science occurs as small bits of evidence accumulate is which lets scientists refine and expand on earlier ideas. The correct option is a.
What is science?Science is the subject of learning facts and ideas about the earth and other natural things present on the earth. It constitutes the phenomena and the acts behind the happening of things. They search and contribute to the reason behind something natural.
Science works based on evidence and proof. It describes the theories and facts behind natural things. So taking baby steps in science is essential to make it clear that no facts are left behind. Science always changes because of the changing of things.
Thus, the correct option is a. let scientists refine and expand on earlier ideas.
To learn more about evidence accumulation, refer to the link:
https://brainly.com/question/14917384
#SPJ2
what is the name of Be(ClO)2
Answers
Answer:
Hypochlorite
Explanation:
In chemistry, hypochlorite is an anion with the chemical formula ClO⁻. It combines with a number of cations to form hypochlorite salts. Common examples include sodium hypochlorite and calcium hypochlorite. The Cl-O distance in ClO⁻ is 210 pm.
Answer:
Barium hypochlorite
Chemical formula: Ba(ClO)₂
Melting point: 235 °C (455 °F; 508 K) (decomposes)
Solubility in water: reacts
hope this helps
The most concentrated solution from among those listed is
Answers
if their was no trees on this planet what would have happen to society
Answers
Answer:
Life could not exist on Earth without trees because they produce most of the oxygen that humans and wildlife breathe. Trees absorb carbon dioxide from the atmosphere and release oxygen using the process of photosynthesis.
Bonds payable to whomever holds them are called _____ bonds or unregistered bonds.
Answers
Bonds payable to whomever holds them are called bearer bonds or unregistered bonds.
What is bearer bond ?Bonds that have no registered owners are known as bearer bonds. Instead, the bond is owned by whoever "bears" (or has possession of) it. Bearer bonds, sometimes known as coupons, have coupons that bondholders can detach and present to receive interest payments.
What are interest payments ?An interest rate is the amount of interest that is payable each period stated as a percentage of the amount that was lent, deposited, or borrowed. The principal amount, interest rate, frequency of compounding, and duration of the loan, deposit, or borrowing determine the total amount of interest on a sum that was lent or borrowed.
To know more about interest visit :
https://brainly.com/question/13324776
#SPJ4
Why would a heavier object require a greater force to accelerate?
Answers
Answer:
An object with more mass (heavier object), requires more force to set it in motion if something neglects friction. Newton's 2nd law, F=ma, tells us that the force required to produce a given acceleration is proportional to the mass of the object.
Explanation:
Hope this helps :)
The combustion of octane, C8H18, proceeds according to the reaction shown.
2C8H18(l)+25O2(g)⟶16CO2(g)+18H2O(l)
If 562 moles of octane combusts, what volume of carbon dioxide is produced at 38.0 °C and 0.995 atm?
=________________L
Answers
First, we need to use stoichiometry to find the moles of carbon dioxide produced. From the balanced equation, we can see that for every 2 moles of octane combusted, 16 moles of carbon dioxide are produced.
So, we can set up a proportion:
562 moles octane / 2 moles octane = x moles CO2 / 16 moles CO2
Solving for x, we get:
x = (562 moles octane / 2 moles octane) * (16 moles CO2 / 1) = 4496 moles CO2
Next, we can use the ideal gas law to find the volume of carbon dioxide produced. The ideal gas law is:
PV = nRT
where P is pressure, V is volume, n is moles, R is the gas constant, and T is the temperature in Kelvin.
We are given the pressure (0.995 atm), temperature (38.0 °C = 311 K), and moles of carbon dioxide (4496). We also need to use the gas constant R, which is 0.08206 L atm/(mol K).
Plugging in these values, we can solve for V:
V = nRT/P = (4496 moles) * (0.08206 L atm/(mol K)) * (311 K) / (0.995 atm) = 118,687 L
Rounding to the nearest whole number, the volume of carbon dioxide produced is 118,687 L.
Learn more about carbon dioxide here:
https://brainly.com/question/3049557
#SPJ11
If you start with 100 grams of hydrogen-3, how many grams will you have after 24.6 years?
Answers
Answer:
The mass left after 24.6 years is 25.0563 grams
Explanation:
The given parameters are;
The mass of the hydrogen-3 = 100 grams
The half life of hydrogen-3 which is also known as = 12.32 years
The formula for calculating half-life is given as follows;
\(N(t) = N_0 \times \left (\dfrac{1}{2} \right )^{\dfrac{t}{t_{\frac{1}{2} }} }\)
Where;
N(t) = The mass left after t years
N₀ = The initial mass of the hydrogen-3 = 100 g
t = Time duration of the decay = 24.6 years
\(t_{\frac{1}{2} }\) = Half-life = 12.32 years
\(N(24.6) = 100 \times \left (\dfrac{1}{2} \right )^{\dfrac{24.6}{12.32}} } = 25.0563\)
The mass left after 24.6 years = 25.0563 grams.
the third law of thermodynamics describes the entropy of a: select the correct answer below: solid liquid gas all of the above
Answers
The third law of thermodynamics describes the entropy of a: solid.
The third law of thermodynamics states that the entropy of a pure crystalline substance approaches zero as the temperature approaches absolute zero (0 Kelvin or -273.15 degrees Celsius). This law implies that at absolute zero, a perfectly ordered and pure crystalline solid will have zero entropy.
The third law of thermodynamics is not specific to liquids or gases but applies to solids. In a solid, the molecules are highly ordered and have fixed positions in a regular lattice structure. As the temperature decreases towards absolute zero, the thermal motion of the molecules reduces, and the system becomes more ordered, resulting in a decrease in entropy.
In contrast, liquids and gases have higher entropy compared to solids at absolute zero because their molecules have more freedom of movement and are not as tightly arranged. Therefore, the third law of thermodynamics specifically addresses the entropy of solids and does not apply to liquids or gases.
To learn more about law of thermodynamics, here
https://brainly.com/question/1368306
#SPJ4
it Test Review
Active
1
2
4
3
7
5
DO
6
"Which statement best explains the change that occurs when gas particles move more slowly?
The temperature increases because the average kinetic energy decreases
O The temperature increases because the average kinetic energy increases
The temperature decreases because the average kinetic energy decreases.
O The temperature decreases because the average kinetic energy increases
Answers
The statement "The temperature decreases because the average kinetic energy decreases" best explains the change that occurs when gas particles move more slowly.
Temperature is a measure of the average kinetic energy of the particles in a substance. When gas particles move more slowly, their average kinetic energy decreases, which means that the temperature of the gas decreases. This is because the particles have less energy to transfer to their surroundings, resulting in a decrease in temperature. Therefore, the correct statement is "The temperature decreases because the average kinetic energy decreases."
When gas particles move more slowly, their kinetic energy decreases. Kinetic energy is directly related to temperature, so as the kinetic energy decreases, so does the temperature. This is in line with the concept of temperature being a measure of the average kinetic energy of the particles in a substance.
To know more about particles visit:
https://brainly.com/question/2288334
#SPJ11