if the specific gravity of the yellow manometer fluid is sg = 0.90 and the deflection is h = 0.7 what is the velocity of the fluid in ft/s
Answers
The velocity of the yellow manometer fluid is 6.44 ft/s.
To find the velocity of the fluid in ft/s, we can use the Bernoulli equation for incompressible flow along a streamline:
P + (1/2)ρv² + ρgh = constant
Where P is the pressure, ρ is the density, v is the velocity, g is the acceleration due to gravity, and h is the height.
Since the pressure on both sides of the manometer is the same, we can simplify the equation to:
(1/2)ρv² = ρgh
Rearranging the equation to solve for v:
v = √(2gh)
We can also use the relationship between specific gravity and density to find the density of the fluid:
sg = ρ/ρₒ
Where sg is the specific gravity, ρ is the density of the fluid, and ρₒ is the density of water (1000 kg/m³ or 62.4 lb/ft³).
Solving for ρ:
ρ = sg(ρₒ)
Substituting the values given in the question:
ρ = 0.90(62.4 lb/ft³) = 56.16 lb/ft³
Now we can plug in the values for ρ, g, and h into the equation for v:
v = √(2)(32.2 ft/s²)(0.7 ft)
v = 6.44 ft/s
Therefore, the fluid has a velocity of 6.44 ft/s.
Learn more about Bernoulli equation here: https://brainly.com/question/15396422.
#SPJ11
Related Questions
please help me and thx
For the periodic table
What is a period?
Define a family / group ?
With own word please and thx
Answers
Answer:
In the Periodic Table A period are the rows on the table.
base ur answers 6 through 13 on the info bellow about elements A,B,C, and D
A(12 protons, 12 neutrons, 12 electrons) B (12 protons, 13 neutrons, 12 electrons) C (12 protons, 12 neutrons, and 11 electrons) D (11 protons, 12 neutrons, 11 electrons)
6) what is the symbol for element A?
7)What is the name of element A?
8) what is the mass number for element A?
9) what is the atomic number for element A?
10) which diagram (B,C, or D) is NOT the same element as element A? Explain
11) which diagram (B,C or D is an ion of element A? Explain
12)What is the charge of the ion you selected in question 11? Explain
13)what diagram (B,C,or D) is an isotopes of element A? Explain
Answers
Answer:
Element Symbol Atomic
Number Atomic Mass Protons Neutrons Electrons
copper Cu 29 64 29 35 29
tin Sn 50 119 50 69 50
iodine I 53 127 53 74 53
uranium U 92 238 92 146 92
potassium K 19 39 19 20 19
lithium Li 3 7 3 4 3
oxygen O 8 16 8 8 8
gold Au 79 197 79 118 79
sulfur S 16 32 16 16 16
silver Ag 47 108 47 61 47
chromium Cr 24 52 24 28 24
cobalt Co 27 59 27 32 27
nickel Ni 28 59 28 31 28
zinc Zn 30 65 30 35 30
aluminum Al 13 27 13 14 13
mercury Hg 80 201 80 121 80
platinum Pt 78 195 78 117 78
iron Fe 26 56 26 30 26
hydrogen H 1 1 1 0 1
helium He 2 4 2 2 2
beryllium Be 4 9 4 5 4
magnesium Mg 12 24 12 12 12
carbon C 6 12 6 6 6
silicon Si 14 28 14 14 14
chlorine Cl 17 35 17 18 17
bismuth Bi 83 209 83 126 83
boron B 5 11 5 6 5
calcium Ca 20 40 20 20 20
manganese Mn 25 55 25 30 25
lead Pb 82 207 82 125 82
sodium Na 11 23 11 12 11
fluorine F 9 19 9 10 9
phosphorus P 15 31 15 16 15
Explanation:
Please answer the questions in the picture below





Answers
Answer b
Explanation:
HELPPPP!!!! SCIENCE!!!!
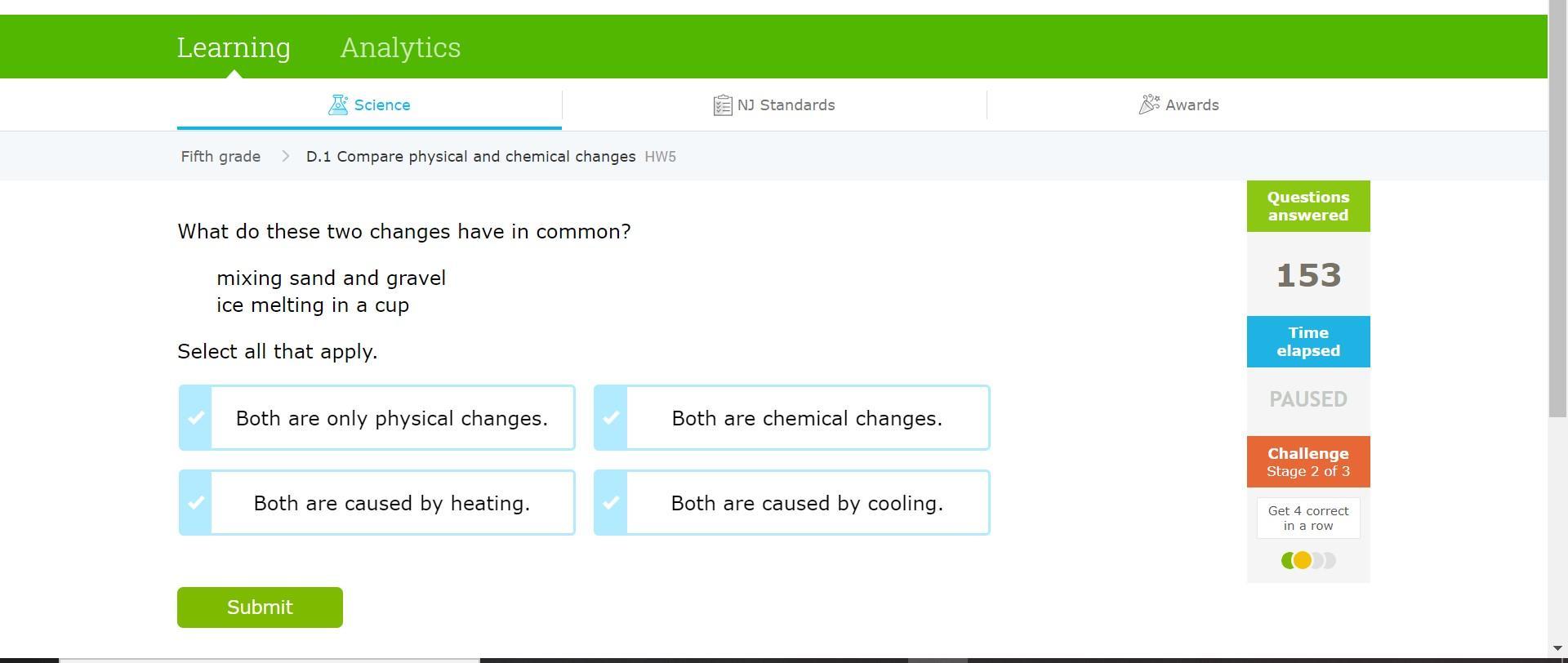
Answers
Answer:
Both are only physical changes
the main site for water reabsorption along the nephron is the __________.
Answers
The main site for water reabsorption along the nephron is the renal tubules
Particularly the proximal tubule and the descending limb of the loop of Henle. As filtrate flows through the renal tubules, water and solutes are selectively reabsorbed into the bloodstream, with the majority of water reabsorption occurring in the proximal tubule. In this region, water is reabsorbed via osmosis, facilitated by the presence of aquaporin channels in the apical and basolateral membranes of the tubule cells. The descending limb of the loop of Henle is also important for water reabsorption, as it is permeable to water but not solutes, allowing for the creation of a concentration gradient that facilitates water reabsorption in the later parts of the nephron.
To know more about nephron, here
brainly.com/question/30975111
#SPJ4
Explain why one molecule NaBH4 will reduce only two moelcules of benzil
Answers
One molecule of NaBH4 can reduce only two molecules of benzil because only two of the four available hydride ions in NaBH4 participate in the reaction. This limitation is due to the selective nature of the reaction and steric hindrance caused by the bulky boron-hydrogen bonds in NaBH4.
Sodium borohydride (NaBH4) is a reducing agent commonly used in organic chemistry.
Benzil is a compound that contains two carbonyl (C=O) groups. When NaBH4 reacts with benzil, it donates a hydride ion (H-) to each carbonyl group, reducing them to alcohols.
One molecule of NaBH4 has four hydrogen atoms attached to the boron atom, and it can donate one hydride ion per hydrogen atom.
However, the reaction with benzil is selective, meaning that only two of the four hydride ions in NaBH4 participate in the reduction of two molecules of benzil. This selectivity is due to the steric hindrance caused by the bulky boron-hydrogen bonds, which prevents the remaining two hydride ions from being utilized in the reaction.
For more such questions on benzil, click on:
https://brainly.com/question/14893972
#SPJ11
The nucleus of a hellum atom Is Identical to:
A.a gamma particle
B.an alpha particle
C.a beta particle
D.all of the above
Answers
Answer:
B
Explanation:
Its nucleus is identical to an alpha particle, and consists of two protons and two neutrons. Alpha decay of heavy elements in the Earth's crust is the source of most naturally occurring helium-4 on Earth, produced after the planet cooled and solidified.
Answer: B. An Alpha particles
As helium is consist of 2 protons and 2 neutrons
Alpha particles, also consist of 2 protons and 2 neutrons bound together into a particle that's why identical to a helium-4 nucleus.
pls help me tell me how to do this question. i only need the answer for the first 2.pls

Answers
Answer:
Explanation:
55 and 918
• Describe how crystals of copper sulfate can be obtained from the salt solution.
Answers
Answer:
Crystals of copper sulfate can be obtained from the salt solution through a process called crystallization. The steps involved are:
Prepare a saturated solution of copper sulfate by dissolving the salt in water until no more can dissolve.
Heat the solution to evaporate some of the water, which will increase the concentration of the salt.
Allow the solution to cool slowly. As it cools, the solubility of the salt decreases and it will start to form crystals.
Use a filter or strainer to separate the crystals from the remaining liquid. This process is called filtration.
Wash the crystals with a small amount of cold water to remove any impurities that may be present.
Leave the crystals to dry on a filter paper or in a drying oven.
After following these steps, pure crystals of copper sulfate can be obtained from the salt solution.
Explanation:
Draw the stracture of 2-bromo-4-chloro-3, 3-dimethylhex-1-ene
Answers
answer :
this is the structure if you want it


When silver carbonate dissolves in water, what ions are produced?
Answers
When silver carbonate (Ag₂C₃) dissolves in water, it dissociates into silver ions (Ag+) and carbonate ions (CO₃ 2-).
When silver carbonate (Ag₂C₃) dissolves in water, it dissociates into its constituent ions. The formula unit of silver carbonate consists of two silver ions (Ag+) and one carbonate ion (CO₃ 2-). When silver carbonate is added to water, the ionic bonds between the silver and carbonate ions are broken, resulting in the release of Ag+ and CO₃ 2- ions into the solution. The Ag+ ions carry a positive charge, while the CO₃ 2- ions carry a negative charge, and these ions become solvated in the water.
Learn more about ions here: brainly.com/question/14982375
#SPJ4
How does the moon's physical characteristics make it easier for scientists to study than the sun?
Responses
A. The moon is closer to Earth so it is easier to see and visit.
B. The moon is solid so it is easier for astronauts to visit.
C. The moon is not as bright as the sun so it is easier to view through a telescope.
D. All of the other answer choices
2.Earth completes one rotation every 24 hours. The moon completes one rotation about every 28 days. How are the day/night cycles different on the moon and on Earth?
Responses
A. The moon's day/night cycle takes 28 days instead of 24 hours.
B. On the moon, it is always daytime.
C. On the moon, it is always nighttime.
D. The moon has a week/month cycle instead of a day/night cycle.
Scientists noticed that climate changes on Earth cycled through about every 11 years. The scientists realized the changes were affected by the sun. Which feature of the sun causes these Earth climate changes?
Responses
A. None of the other answer choices
B. Solar flares
C. The corona
D. Sunspots
Answers
Answer:
1st question: The moon is closer to Earth so it is easier to see and visit.
2nd question: The moon has a week/month cycle instead of a day/night cycle.
3rd question: The corona
Explanation:
How many grams are there in 2.34x10^23 atoms of Cu?
Answers
Explanation
Because it’s 1
The mass of the 2.34 × 10²³ atoms of copper is equal to 24.68 grams.
What is Avogadro's number?The Avogadro constant can be defined as the proportionality factor that the number of constituent particles in a sample with the amount of substance.
Avogadro’s number can be described as a dimensionless number that represents the number of entities in one mole of any substance. These elementary entities can be molecules, atoms, ions, electrons, or protons, etc.
Avogadro’s constant has a value approximately equal to 6.022 × 10²³ mol⁻¹.
Given, the number of atoms of copper = 2.34 × 10²³
The atomic mass of Cu Copper = 63.5 g/mol
So 6.022 × 10²³ atoms of Cu have mass = 63.5 g
Then 2.34 × 10²³ Cu atoms will have mass = \(\frac{63.5 \times 2.34 \times 10^{23}}{6.02 \times 10^{23}}\) = 24.68 g
Therefore, 24.68 grams of Cu are there in 2.34 × 10²³ atoms of Cu.
Learn more about Avogadro's number, here:
brainly.com/question/11907018
#SPJ2
At STP, the volume of N2(g) produced by the complete decomposition of 1 mole of nitroglycerin would be closest to which of the following?
A.5 L
B.10 L
C.20 L
D.30 L
Answers
To determine the volume of N2(g) produced by the complete decomposition of 1 mole of nitroglycerin (C3H5N3O9), we need to consider the balanced chemical equation for the decomposition reaction.
The balanced equation for the decomposition of nitroglycerin is as follows:
4 C3H5N3O9(s) → 12 CO2(g) + 10 H2O(g) + 6 N2(g) + O2(g) From the balanced equation, we can see that for every 4 moles of nitroglycerin, 6 moles of N2(g) are produced. Since we are considering the decomposition of 1 mole of nitroglycerin, we can use this ratio to determine the moles of N2(g) produced, which is 6/4 = 1.5 moles of N2(g). Now, at STP (Standard Temperature and Pressure), 1 mole of any ideal gas occupies 22.4 liters. Therefore, 1.5 moles of N2(g) would occupy approximately 33.6 liters
Learn more about decomposition reaction here: brainly.com/question/2285034
#SPJ11
Calculate the mass of water produced when 25.0g of oxygen gas react with
42.0g of hydrogen gas.
2H2 + O2 -> 2H,0
Answers
Reaction.-
2H2 + O2 ---> 2H2O
Given,
Mass of Oxygen - 25.0 g
Mass of Hydrogen - 42.0 g
now we have to calculate moles
moles of O2 = 25/16 = 1.5625 moles
Moles of H2 = 42/2 = 21 moles
Hydrogen gas is in excess,
now using reaction
1 mole of oxygen gas forms 2 moles of H2O
1.5625 mole of Oxygen gas will form x mole of H20
x = 3.125 moles
molar mass of H2O is 18g
mass of 3.125moles of water = 3.125 × 18 = 56.25 g.
Hence the mass of water produced when 25.0g of oxygen gas react with 42.0g of hydrogen gas is 56.25 grams
How long will it take a piece of strontium-90 (produced in a nuclear test blast) weighing exactly 1000.0 g to be
reduced to 15.625 g? The half-life of strontium 90 is 28.8 years,
Answers
Answer:
Time taken 172.8 years
Explanation:
Given data:
Total amount of strontium-90 = 1000.0 g
Amount left = 15.625 g
Half life of strontium = 28.8 years
Time taken = ?
Solution:
Number of half lives:
At time zero = 1000.0 g
At first half life = 1000.0g/2 = 500 g
At second half life = 500 g/2 = 250 g
At third half life = 250 g/ 2 = 125 g
At 4th half life = 125 g/ 2= 62.5 g
At 5th half life = 62.5 g/2 = 31.25 g
At 6th half life = 31.25 g / 2 = 15.625 g
Time taken:
Time taken = number of half lives × half life
Time taken = 6×28.8 years
Time taken = 172.8 years
PLSSS HEALP ASAP!!!! WILL REWARD
A) How many moles of CO will react with 1.75 moles of Fe2O3?
B) What was the mole ratio of CO to Fe2O3?
Answers
A)1.75×3 moles of carbon monoxide
B)2:3
A)each mole of ferric oxide requires 3 moles of carbon monoxide. Therefore 1.75 moles requires 1.75 ×3 moles of carbon monoxide
which of the following is a characteristic of the modern periodic table
Answers
Which of the following are examples of a physical property of matter? Select all that apply.
Answers
The matter is made up of various types of particles which have a physical state and have inertia.
What are the physical property of matter? Select all that apply.1. Matter is colour less. (false)
2. Matter is hard in structure. (true)
3. Matter is soluble. (true)
4. Matter can not conduct electricity. (false)
5. Matter has boiling point but not melting point. (false)
6. Matter has both boiling and melting point. (true)
7. Matter has not the density. (false)
8. Matter has the malleability. (true)
Matter is made up of different kinds of particles which can occupy space and they have some mass. All the matters are made up of the elements. The elements contain specific physical and chemical properties. the matter can not be broken by any ordinary chemical reaction.
So we can conclude that the matter is made up of various types of particles which have a physical state and have inertia.
Learn more about Matter here: https://brainly.com/question/3998772
#SPJ1
In an experiment, 10.6 grams of steam is produced and then cooled. If the heat of vaporization is 2,257 joules/gram, how much energy is released after all the vapor turns to liquid?
Answers
Answer:
23,900 joules
Explanation:
plato answer
Answer:
23,900 joules
Explanation:
Edmentum
what does Le châteliers principle state?
Answers
Hope this helps!
8. Some people practice brining their turkey. This means they let it soak in a solution overnight. The solution diffuses into the turkey. This means that the turkey is placed into a ___________________ solution.
Answers
Some people practice brining their turkey. This means they let it soak in a solution overnight. The solution diffuses into the turkey. This means that the turkey is placed into a hypertonic solution.
What is a hypertonic solution?A hypotonic solution is described as a solution that has lower osmotic pressure than another solution to which it is compared.
The concept of tonicity helps us understand that the saltwater we use to brine the turkey is typically considered hypertonic solution because it has a greater concentration of solutes than the liquid found inside the cells.
Learn more about hypertonic solutions at: https://brainly.com/question/4237735
#SPJ1
How do you figure out what roman numeral to use? Give a specific example
Answers
Answer:Explanation:
The Roman numeral must have the same value as the charge of the ion. In our example, the transition metal ion Fe2+ would have the name iron(II). Add the name of the anion to the transition metal ion. In our example, FeCl2 would have the name iron(II) chloride since the anion is Cl-, which has the name chloride.
can anybody help me with a question in chemistry?
Answers
What is the density of object B? Does it sink or float in water?

Answers
Answer:
The density of B is 11/14 = 0.79 g/cm³
It will float in water
What reagent could you add to a mixture of Mn+2(aq) and Zn+2(aq) to separate the two species?
a. NaOH b. HzDMG c. NaBiO3 d. BaClz e. KaFe(CN)s
Answers
"Group analysis" is a well-known qualitative analysis method that is used with NaBiO3 for this objective. Its distinctive characteristics are by subjecting a mixture of cations to a series of reagent treatments.
Zinc (Zn) is more quickly oxidised than manganese (Mn)?Zn has a larger negative reduction potential than Mn. As a result, if we add a potent oxidising agent, Mn+2 will remain unaffected and Zn will be preferentially oxidised to its +2 oxidation state. We may then segregate the two species as a result.
The sole powerful oxidising agent among the reagents given is (c) NaBiO3 (sodium bismuthate).As a result, we can separate the two species by adding NaBiO3 to the solution of Mn+2(aq) and Zn+2(aq). Zn+2 will be converted by the NaBiO3 to Zn(OH)4 and precipitated out of the solution, but Mn+2 will remain in the solution as Mn(OH)2. Zn+2 cannot be oxidised by the other specified reagents since they lack potent enough oxidising abilities.
to know more about reagents here:
brainly.com/question/31228572
#SPJ1
Look at the reaction below and state which direction the reaction would shift:
A closed container of water and its vapor at equilibrium. Vapor is added to the system.
Water + Energy <=> Vapor
Answers
A system's equilibrium will move to the right, or toward the side of the products, in accordance with Le Chatelier's principle, if more reactants are added. ... The equilibrium will move to the left if we add more product to a system, producing more reactants.
What causes the rightward tilting of equilibrium?Solution: By increasing the number of reactants, the equilibrium moves to the right and in the direction of the products.
What causes the balance to tilt to the left?Thus, if a reactant is added, equilibrium shifts to the right, away from the reactant. Equilibrium shifts to the left, away from the product, when a product is added. If we take away the product, equilibrium returns and produces the product. Reactant is created if reactant is removed, breaking the equilibrium.
To know more about Le Chatelier's principle visit :-
https://brainly.com/question/29009512
#SPJ1
chemistry help please

Answers
Answer:
the right answer is first one
What is the total number of sulfur atoms in Mg2(SO4)3
Answers
AnswerElement Symbol # of Atoms
Magnesium Mg 1
Oxygen O 4
Sulfur S 1
:
Explanation:
How many significant figures are in this measurement: 1,250,500