if 0.246 g of mg are reacted with 10.0 ml of 3.00 m hcl, how many moles of h2 should be produced theoretically?
Answers
The theoretical yield of hydrogen gas in this reaction is 0.00505 moles. To solve this problem, we need to first write out the balanced chemical equation for the reaction between magnesium and hydrochloric acid:
Mg + 2HCl → MgCl2 + H2
From the equation, we can see that one mole of magnesium reacts with two moles of hydrochloric acid to produce one mole of hydrogen gas.
Next, we need to calculate the number of moles of magnesium in 0.246 g of Mg:
molar mass of Mg = 24.31 g/moles of Mg = mass / molar mass = 0.246 g / 24.31 g/mol = 0.0101 mol
Now we can use the molarity of the hydrochloric acid and the stoichiometry of the balanced chemical equation to calculate the number of moles of hydrogen gas produced:
molarity of HCl = 3.00 mol/L
volume of HCl = 10.0 mL = 0.0100 L
moles of HCl = molarity × volume = 3.00 mol/L × 0.0100 L = 0.0300 mol
moles of H2 = 0.5 × moles of Mg = 0.5 × 0.0101 mol = 0.00505 mol
Therefore, the theoretical yield of hydrogen gas in this reaction is 0.00505 moles.
For more information on chemical equation see:
https://brainly.com/question/30087623
#SPJ4
Related Questions
Which of the following is the equation for wave speed?
Answers
Answer:
\({ \boxed{ \mathfrak{ \: answer : \: { \bf{v = f \lambda}} }}}\)
v is the wave speedf is frequencylambda is wave lengthWhich of the following cannot be classified as a mixture
A. Stainless steel
B. Rubbing alcohol
C. Table salt (NaCI
D. Granite
Answers
Answer:
B) Rubbing alcohol
Explanation:
It is a solution
how can i solve this using dimensional analysis im so confused

Answers
Answer:follow these steps Identify the given quantity in the problem.
Identify the wanted quantity in the problem.
Establish the unit path from the given quantity to the wanted quantity using equivalents as conversion factors.
Set up the conversion factors to permit cancellation of unwanted units
Explanation:
other things that may helps
Set up each problem by writing down what you need to find with a question mark. Then set it equal to the information that you are given. The problem is solved by multiplying the given data and its units by the appropriate unit factors so that only the desired units are present at the end.
Answer:
I will answer the first one for you: 5.33
Explanation:
You can use dimensional analysis to do conversions in almost any field of math. For the first problem:
\(\frac{16 feet}{} |\frac{1 meter}{3 feet} = about 5.33\)
Carbon cycle – What are the main reservoirs
of the carbon cycle? Where do the inorganic and organic carbon
cycles interact? What are the major differences and similarities
between the inorganic and organic carbon?
Answers
The main reservoirs of the carbon cycle are the atmosphere, oceans, land (including vegetation and soils), and fossil fuels. In these reservoirs, carbon exists in both inorganic and organic forms.
The inorganic carbon cycle involves the exchange of carbon dioxide (CO2) between the atmosphere and oceans through processes like photosynthesis and respiration.
Organic carbon, on the other hand, is found in living organisms, dead organic matter, and soil organic matter. It is cycled through processes such as decomposition and consumption by organisms. The interactions between the inorganic and organic carbon cycles occur primarily in the biosphere, where photosynthesis converts inorganic carbon into organic carbon compounds. While inorganic carbon is primarily in the form of CO2, organic carbon is present in complex organic molecules. Both forms of carbon play crucial roles in energy transfer, nutrient cycling, and climate regulation.
Learn more about Carbon Cycle
brainly.com/question/13729951
#SPJ11
This atom can form up to _____ single covalent bond(s). An atom with two electron shells. There is one electron pair in the inner electron shell and four unpaired electrons in the outer electron shell. 0 1 4 2 3
Answers
Answer:
4
Explanation: it's a carbon atom right....soo it'll form 4 bonds(single)
An atom can form 4 single covalent bonds is carbon with two electron shell.
What are covalent bonds?The bonds are defined as a strong bond that binds atoms, ions, or molecules together and promotes the production of molecules.
There are three types of bonds.
Ionic bondCovalent bondMetallic bondCovalent bonds are defined as the bond formed by sharing of electrons with each other.
Covalent bonding is a stable equilibrium of the attractive and repulsive forces between two atoms that occurs when they share electrons. Bonding pairs or sharing pairs are other names for these electron pairs.
It can also be defined as a type of chemical link where atoms share electrons to create electron pairs.
Covalent bonds has low melting point, boiling point, enthalpy of fusion and enthalpy of vaporization.
Thus, an atom can form 4 single covalent bonds is carbon with two electron shell.
To learn more about covalent bonds, refer to the link below:
https://brainly.com/question/10777799
#SPJ12
The image above shows the inner planets which plan is represented by the number two?
Answers
Answer:
Venus
Explanation:
From the sun outwards is Mercury Venus Earth and then Mars
how do you separate a heterogenous mixture that contains powdered sulfur, pebbles, powdered iron, sand, and magnesium sulfate??
Answers
Identify the definition that applies to the compound in red. F-(aq) HSO4-(aq) → HF(aq) SO42-(aq) Arrhenius acid Bronsted-Lowry acid Arrhenius base Bronsted-Lowry base
Answers
The appropriate definition that applies to the compound in red (F-(aq)) is Bronsted-Lowry base.
How to determine the definition that applies to the compound in red. F-(aq) HSO4-(aq) → HF(aq) SO42-(aq) Arrhenius acid Bronsted-Lowry acid Arrhenius base Bronsted-Lowry baseBased on the given chemical equation F-(aq) + HSO4-(aq) → HF(aq) + SO42-(aq), the compound in red is F-(aq) (fluoride ion).
In this context, F-(aq) acts as a base by accepting a proton (H+) from the HSO4-(aq) ion, forming HF(aq) and SO42-(aq).
Therefore, the appropriate definition that applies to the compound in red (F-(aq)) is Bronsted-Lowry base.
In the given chemical equation F-(aq) + HSO4-(aq) → HF(aq) + SO42-(aq), the compound highlighted in red is F-(aq), which represents the fluoride ion.
In this specific context, F-(aq) demonstrates its characteristics as a base by accepting a proton (H+) from the HSO4-(aq) ion. This proton transfer reaction results in the formation of HF(aq) and SO42-(aq).
Therefore, based on its behavior in the equation, the appropriate definition for the compound in question, F-(aq), is that of a Bronsted-Lowry base.
Learn more about chemical equation at https://brainly.com/question/11231920
#SPJ1
N-Butane is converted to isobutane in a continuous isomerization reactor that operates isothermally at 149°C. The feed to the reactor contains 93 mole% n-butane, 5% isobutane, and 2% HCl at 149°C, and a 40% conversion of n-butane is achieved. Calculate the amount of heat transfer (kJ). Use a basis of 1 mol total feed
Answers
n-Butane is transformed to iso-butane at excessive temperatures withinside the presence of an acid. The volume of isomerization is decided via way of means of calculating the distinction withinside the wide variety of moles of additives all through the response.
n-Butane is transformed to isobutane in a non-stop isomerization reactor that operates isothermally at 142°C. The feed to the reactor consists of ninety three mole% n-butane, 5% isobutene, and 2% HCl at 142°C, and a 36% conversion of n-butane is achieved. Taking a foundation of one mol of feed gas, calculate the moles of every aspect of the feed and product combos and the volume of response, ξ (mol).
Calculate the same old warmness of the isomerization response (kJ/mol). Then, taking the feed and product species at 25 °C as references, put together an inlet-outlet enthalpy desk and calculate and fill withinside the aspect amounts (mol) and particular enthalpies (kJ/mol). (See Ex 9.5-1 for your textbook.)
Calculate the desired fee of warmth switch (kJ) to or from the reactor (nation which it is). Then decide the desired warmness switch fee (kW) for a reactor feed of 325 mol/h. Use your calculated consequences to decide the warmth of the isomerization response at 149 °C, (142 °C)(kJ/mol).
Learn more about N-Butane here https://brainly.com/question/6328164
#SPJ4
How does anaerobic respiration occur in yeast ?
Answers
Allowing NADH to lose hydrogen allows it to be converted to NAD, which can then be utilized to oxidize glucose to pyruvate, which produces ATP, and so on. This is best illustrated in a diagram, in my opinion.
An excess of a divalent metal M was dissolved in a limited volume of hydrocloric acid. If 576cm³ of hydrogen was librated at s.t.p , what was the mass of metal that produced this volume of gydrogen?( M = 24, H = 1 , molar volume of gas at s.t.p = 22.4dm³)
Answers
Answer:
14.8 g
Explanation:
step 1 You first have to convert the cm^3 to dm^3 by dividing the volume by 1000 then find the number of mols for Hydrogen gas
from there you can use the of moles for hydrogen to find the moles of the metal step 2
then step 3 you multiply the number of by The Mr or Fr giving you your mass

Rank the following compounds in order of increasing boiling point.
(a) CH3CH2CH2OCH2CH3CH3CH2CH2OCH2CH3
(b) (CH3CH2)2CHOH(CH3CH2)2CHOH
(c) (CH3)2CHCH2CH2OH
Answers
The following compounds can be ranked in the order of increasing boiling point as (a) < (b) < (c).
The boiling point depends upon the intermolecular forces present in the molecule. In general, the stronger the intermolecular forces, the higher the boiling point. Here, we are going to rank the following compounds in order of increasing boiling point. Rank the following compounds in order of increasing boiling point (a) CH3CH2CH2OCH2CH3CH3CH2CH2OCH2CH3 (b) (CH3CH2)2CHOH(CH3CH2)2CHOH (c) (CH3)2CHCH2CH2OH.
The compounds can be ranked in order of increasing boiling points as follows: (a) CH3CH2CH2OCH2CH3CH3CH2CH2OCH2CH3, there are two ether groups in the molecule, so the only intermolecular force present is London Dispersion force, it is the weakest of all intermolecular forces. Hence, the boiling point is the lowest. (b) (CH3CH2)2CHOH(CH3CH2)2CHOH, this molecule contains hydrogen bonding which is a stronger intermolecular force than London dispersion force. So, the boiling point is higher than that of (a). (c) (CH3)2CHCH2CH2OH, this molecule has both hydrogen bonding and London Dispersion forces present. Hence, the boiling point is the highest among all. The compounds can be ranked in the order of increasing boiling point as (a) < (b) < (c).
Learn more about boiling point at:
https://brainly.com/question/16945842
#SPJ11
Why do lungfish walk on land?
Answers
Lungfish walk on land to suck in atmospheric air.
What are lungfish?The Dipnoi are a group that lives exclusively in freshwater environments. Its main feature is breathing through a structure that works like a primitive lung. This structure is highly vascularized and is linked to the pharynx.
With this information, we can conclude that the lungfish leave the water to breathe atmospheric air.
Learn more about Fish in https://brainly.com/question/25039796
Where is the potential energy to run the plant?
A 3
B
4
С
1
D
2

Answers
Answer: C
Explanation:
your well wisher
In 3–5 sentences, explain how meteorologists use weather data to predict the probability of a catastrophic wildfire.
MAKE YOUR OWN DO NOT STEAL FROM ANY WHERE ELSE ALSO MAKE IT AT A MIDDLE SCHOOL LEVEL MAKE THE GRAMMER BAD IF YOU WANT TO PLS AND THANK YOU
Answers
To estimate the likelihood of a catastrophic wildfire, meteorologists utilize meteorological data. To forecast the likelihood of wildfires under several possible climatic scenarios, scientists develop computer models. Scientists forecast where and when wildfires are most likely to occur using various estimates of temperature and precipitation.
Other widely utilized technologies by many authorities and sectors to monitor and identify wildfire outbreaks include terrestrial, aerial, and satellite remote sensing systems as well as IoT-based solutions. To actively search the terrain for fresh flames, terrestrial camera systems have been implemented in numerous areas.
Even though it is not always feasible to identify the particular cause or spark of a fire occurrence, wildfire risk may be roughly calculated by comprehending how climate, geography, weather, and land cover affect fire behavior and the possibility of a fire spreading.
Learn more about prediction on wildfire here https://brainly.com/question/30029887
#SPJ4
I used a stick of (vegan) butter as our example of thinking about how temperature changes the properties of something when it is wamer or cooler.
1. What is something else you could use to describe how temperature affects the properties of when it is warmer or cooler? How does this change whether it is warm or cold. Describe it.
Answers
A gas has a pressure of 2.36 kPa at 62 °C. What is the pressure at standard
temperature?
Answers
Answer:
P2 = 1.94 kPa
Explanation:
Given the following data;
Initial pressure = 2.36 kPa
Initial temperature = 62°C
Standard temperature = 0°C
Conversion:
Kelvin = 273 + C
Kelvin = 273 + 62 = 335 K
Kelvin = 273 + 0 = 273 K
To find the final pressure, we would use Gay Lussac's law;
Gay Lussac states that when the volume of an ideal gas is kept constant, the pressure of the gas is directly proportional to the absolute temperature of the gas.
Mathematically, Gay Lussac's law is given by;
\( PT = K\)
\( \frac{P1}{T1} = \frac{P2}{T2}\)
Making P2 as the subject formula, we have;
\( P_{2}= \frac{P1}{T1} * T_{2}\)
\( P_{2}= \frac{2.36}{335} * 273 \)
\( P_{2}= 0.0071 * 273 \)
P2 = 1.94 kPa
Magnesium reacts with fluorine to form magnesium fluoride. Which diagram shows the correct arrangement of electrons in the product?

Answers
Answer:
Diagram B shows the correct arrangement of electrons in the product.
Magnesium reacts with fluorine to form magnesium fluoride two electrons of magnesium are transferred to the 2 atoms of fluorine and fluorine is the most electronegative element of the periodic table. Option C is correct.
What is electronegativity?Electronegativity is the tendency or power of an atom to attract the electrons from the metals they are mostly nonmetals and want to complete their octave too.
The most electronegative elements of the periodic table are oxygen, nitrogen, and fluorine, as they are always willing to complete the octave, and fluorine will take electrons from magnesium.
Therefore, Option C is correct if the 2 atoms of fluorine and fluorine are the most electronegative element of the periodic table. Magnesium reacts with fluorine to form magnesium fluoride two electrons of magnesium are transferred.
Learn more about electronegativity, here:
https://brainly.com/question/17762711
#SPJ6
How many molecules of co2 are in a 500. 0 ml container at 780 mm hg and 135°c? 8. 76 × 1021 molecules 9. 23 × 1021 molecules 5. 50 × 1021 molecules 2. 65 × 1022 molecules 2. 79 × 1022 molecules.
Answers
Step 1:
ok we have to use the formula PV=nRT
p=Pressure (must be converted to atm)= 780 mmHg
1 amt= 760 mmHg use this as a conversion factor
780 mmHg (1 atm/760 mmHg)= 1.026
V= Volume= 5.00 mL = o.5 L
n=number of moles which we have to find first
R= 0.0821
T(convert to Kelvins by adding 273.15 to the celsius temperature)= 135 C + 273.15= 408.15 k
Now plug in->
(1.026 atm)(o.5 L)= n(0.0821)(408.15 K)
(1.026 atm)(0.5 L)= n(33.509115)
(0.513)= n(33.509115)
n(number of moles)= 0.01532 mol
Now we have to convert to moles using Avagodro's number which states that 1 mol = 6.022 x 10^23 molecules or atoms
So 0.01532 mol (6.022 x 10^23 number of molesules)/ (1 mol) = 9.225704 x 10^21 = 9.226 x 10^21 colecules
Step 2
You must transfer pressure into pascals, 780 mm Hg = 103991 Pa
135*C = 408.15 k
then from the equation pV = nRt
n = pV / RT (T in Kelvins, V in M^3)
n = 103991 x 500 x 10^-6 / (8.314 x 408.15)= 0.015322 moles of N2
1 mol of everything is 6.022 x 10^23 particles, so 0.15322 moles is 0.15322 x 6.022 x 10^23 = 9.2269084 x 10^21 molecules
Explanation:
Hope this helps :)
What are the number of protons, electrons, and neutrons in one atom of neon?
protons
electrons
neutrons
Answers
Answer:
protons- 10
electrons- 10
neutrons- 10
Explanation:
no of protons/ electrons = atomic number
no of neutrons = atomic mass - atomic number
Read through the literacy task
find the mistakes and rewrite
Find and highlight the 10 mistakes
Atoms are the smallest particle that make up living things, they are made up of subatomic particles; protons, neutrons and electrons. Of these the electrons and neutrons are in the nucleus and the protons orbit around the nucleus. Of the subatomic particles, the electrons have the largest mass and are negatively charged, the neutrons (negative charge) and protons (positively charged) both have a relative mass of 1. Atoms contain an equal number of protons and neutrons, so carry no overall charge. An element is a substance made up of only two types of atom. Elements can be found in the Periodic table of elements, they usually have two numbers next to the chemical symbol, the larger number is generally the atomic number, which represents the number of neutrons and the number of protons. The smaller number is the relative atomic mass which represents the number of electrons or neutrons. In order to calculate the number of neutrons, you minus the atomic number from the relative atomic mass.
Answers
Answer:
Atoms are the smallest particle that make up living both living and non living things, they are made up of subatomic particles; protons, neutrons and electrons. Of these the electrons protons and neutrons are in the nucleus and the protons electrons orbit around the nucleus. Of the subatomic particles, the electrons have the largest smallest mass and are negatively charged, the neutrons (negative charge) (no charge) and protons (positively charged) both have a relative mass of 1. Atoms contain an equal number of protons and neutrons electrons, so carry no overall charge. An element is a substance made up of only two one type of atom. Elements can be found in the Periodic table of elements, they usually have two numbers next to the chemical symbol, the larger number is generally the atomic mass number, which represents the number of neutrons and the number of protons. The smaller number is the relative atomic mass atomic number which represents the number of electrons or neutrons protons. In order to calculate the number of neutrons, you minus the atomic number from the relative atomic mass.
What is the density of 10. 002 g of water at 20°c in the units of g/ml?.
Answers
Answer:
0.998 g/mL
Explanation:
https://brainly.com/question/24563794
Can someone plz help me? :(

Answers
Answer:
C.
Explanation:
The toxins get released, as they poison and damage your body's cells. They also damage your lungs, heart, and veins that are inside the body.
hope this helps.
How many moles of gas are in a 30 liter compressed air tank if the
temperature of the tank is 300K and the pressure is 200 atmospheres? *
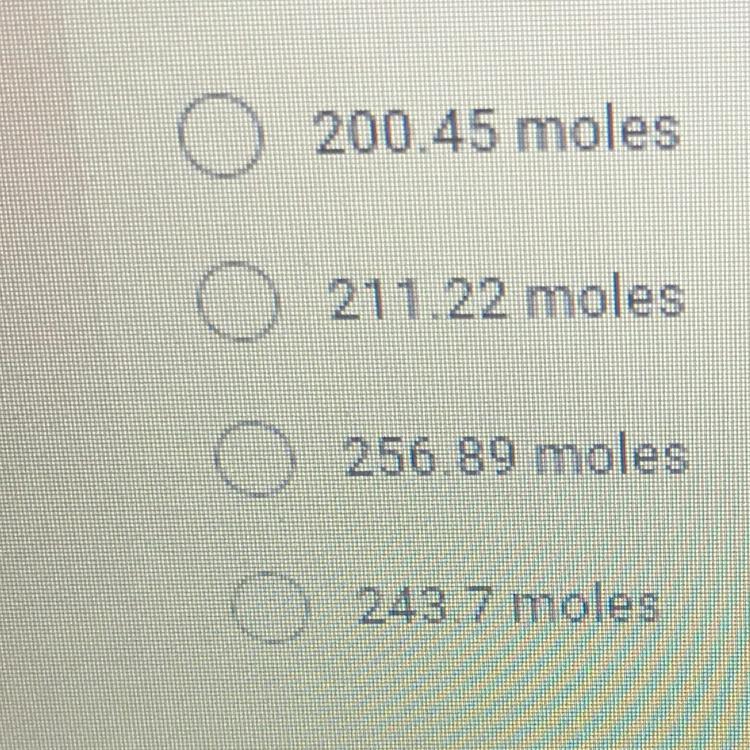
Answers
Answer:
B
Explanation:
Calculate the equilibrium constant for the reaction 2A + 3B -><- C + D if 2.0 moles of A and 3.00 moles of B are introduced into a 2.00L reaction vessel, and allowed to come to an equilibrium at which point 0.400 mol of A remain.
Answers
The equilibrium constant for the reaction is 28.44.
The equilibrium constant expression for the reaction is:
\(Kc = [C][D]/([A]^2[B]^3)\)
where [A], [B], [C], and [D] are the molar concentrations of the respective species at equilibrium.
At the start of the reaction, the initial concentrations of A and B are:
[A] = 2.0 moles / 2.00 L = 1.0 M
[B] = 3.0 moles / 2.00 L = 1.5 M
At equilibrium, the concentration of A is 0.400 mol / 2.00 L = 0.200 M.
We can use these initial and equilibrium concentrations to find the equilibrium concentration of C and D:
[C] = [D] = (2.0 - 0.400) mol / 2.00 L = 0.800 M
Now we can substitute these concentrations into the equilibrium constant expression:
\(Kc = [C][D]/([A]^2[B]^3)\)
\(= (0.800 M)^2 / (0.200 M)^2 (1.5 M)^3\)
= 28.44
For more question on equilibrium click on
https://brainly.com/question/19340344
#SPJ11
A balloon is filled with warm air and placed inside a freezer. After a few minutes, the balloon shrinks. Which of the following most likely happened during the transition?
Owarmer air inside the balloon pushes on the walls of the balloon and the air escapes into the surroundings.
O The warm air molecules inside the balloon loose energy to the cold air molecules, they move slower, and condense
OAs the temperature drops, the warm air molecules gain energy and become more attracted to each other.
O The balloon transfers energy to the cold air, and the cold air gains energy to compress the balloon
Answers
Answer: Option: B - The warm air molecules inside the balloon lose energy to the cold air molecules, they move slower, and condense.
Explanation: Warm air molecules move faster than cold air molecules.
question 38 17) a catabolic pathway may be which of the following? a) a set of reactions that combine monomers into larger, more energy-rich polymers b) a set of coupled reactions that are endergonic c) a set of reactions that form covalent bonds between molecules to store free energy d) a set of reactions that release energy that can be used to drive cellular work
Answers
A catabolic pathway is a d) a set of reactions that release energy that can be used to drive cellular work
A catabolic pathway involves a series of reactions that break down complex molecules into simpler ones, releasing energy in the process. This energy is then utilized by the cell to perform various tasks. Option d correctly identifies a catabolic pathway as a set of reactions that release energy, which can be used to drive cellular work.
During catabolism, large molecules such as carbohydrates, proteins, and fats are broken down into smaller molecules like glucose, amino acids, and fatty acids.
These molecules are then further degraded through oxidation reactions, releasing energy in the form of ATP (adenosine triphosphate). ATP serves as the primary energy currency in cells, providing energy for various cellular processes.
By breaking down complex molecules and releasing energy, catabolic pathways enable cells to obtain the necessary energy to carry out essential functions like metabolism, growth, and movement. So d is correct option.
For more questions like Catabolic pathway click the link below:
https://brainly.com/question/29603008
#SPJ11
Which of the following statements holds true for a chemical change?
A.
In a chemical change, no new substance is formed.
B.
In a chemical change, a substance changes its phase.
C.
In a chemical change, the molecular structure of a substance changes.
D.
In a chemical change, one substance dissolves into another.
Answers
I don't understand how and why some transition metals form more than one ions eg: copper forms two. PLS EXPLAIN. I WILL GIVE YOU BRAINLIEST.PLS EXPLAIN PROPERLY AND IN FULL DETAIL. I AM IN YEAR 9.
THANKYOU
USE COPPER OXIDE IONS AS AN EXAMPLE.
7-8 POINTS
Answers
Answer:because some have more radiation than others causing mutations and there for either decreasing or increasing the amount of ions
copper is a acidic metal in general therefor it will most likely erode faster than any other metal making it hard to determan the number of ions in general the metal will have basicly what I’m trying to say is there is no solid answer as many metals can change there ions for no given reason
Explanation:
what is the limiting reagent when 16 grams of ch4 is reacted with 64 grams of o2? what remains after the reaction?
Answers
CH4 is limiting reagent and H20 remains after the reaction.
What is limiting reagent?A limiting reagent is a reactant that entirely consumes itself before any other reactants are used up in a chemical reaction, hence restricting the amount of product that may be created. It can be found by comparing the proportions of each component in the reaction and its stoichiometry. Once the limiting reagent has been determined, the amount of product that can be generated can be computed using the reaction's stoichiometry.
\(CH_4+2O_2- > CO_2+2H_2O\)
number of moles of CH4= given mass/atomic mass
=> 16/16
so the number of moles of CH4 is 1
number of moles of O2 = 64/32
=2
so CH4 1 mole get vanished after the reaction and the gases like CO2 and O2 fly away so the limiting reagent is CH4
To know more about limiting reagent click below:
https://brainly.com/question/11848702#
#SPJ4