Answers
Answer:
Na,Ba,Ti on the same base placement on the periodic table.
Answer:
O,S,Se
Explanation:
Because they lose electrons and become an anion to become ionicly bonded and they are in the same period
Related Questions
Which of the following properties tells the most about the stability of a metal?
A. Oxidation state
B. Electronegativity
C. Molar mass
D. Atomic number
Answers
Answer: B Electronegativity
(A-p-e-x)
B. Electronegativity tells the most about the stability of a metal.
What is Electronegativity?Electronegativity directs to the ability of an atom to attract the shared electrons in the covalent bond. The more increased the value of the electronegativity is, the more intensely that element attracts the shared electrons.
Electronegativity, symbolized as χ, stands for the tendency for an atom of a provided chemical element to attract shared electrons when creating a chemical bond. An atom's electronegativity is influenced by both its atomic number and the distance at which its valence electrons reside from the charged nucleus.
To learn more about Electronegativity refer to:
https://brainly.com/question/26436343
#SPJ2
What type of reaction is shown below:
Al₂O3 (s) → Al(s) + +O₂(g)
O Acid/Base Neutralization
O Synthesis
O Double Replacement
O Decomposition
O Single Replacement
O Combustion
Answers
Answer: Decomposition
Explanation:
The compound, (Aluminum Oxide), decomposes to form Aluminum and Oxygen.
Zahara Noor wants to create a presentation of different molecules that helped change the world, but she needs help in naming them, so that anyone is able to understand the molecules that she is talking about. Provide the name of the molecule described here:H2O2: an important compound that comes in a liquid form at room temperature, and used in wound cleaning, and disinfecting.
Answers
H2O2 is hydrogen peroxide.
We can determine its name by the function O2(-2):
\(O^{-2}_{^{^{}}2}_{}\)which has the name "peroxide". Since it is combined with hydrogen, we have hydrogen peroxide.
Write a balanced nuclear equation for the beta decay of 234/90Th

Answers
The balanced nuclear equation for the beta decay of 234/90Th can be represented as follows: ^234/90Th --> ^234/91Pa + e^0/-1β
In this equation, the nucleus of thorium-234 (234/90Th) undergoes beta decay. During beta decay, a neutron within the nucleus is converted into a proton, resulting in the emission of an electron (beta particle). As a result, the thorium-234 nucleus is transformed into protactinium-234 (234/91Pa) by gaining one proton.
The beta particle emitted during the decay process is represented as e^0/-1β, where the superscript 0 denotes that the electron has no charge (neutral), and the subscript -1 indicates that the electron carries a negative charge of -1.
It is important to note that in a nuclear equation, the total atomic mass and atomic number on both sides of the equation must be equal to maintain a balanced equation and conserve mass and charge.
For more such questions on balanced nuclear equation visit:
https://brainly.com/question/31505524
#SPJ8
How many joules of heat are needed to melt 50 g of ice at 0°C and then warm the liquid to 65°C? heat of fusion of ice = 334 J/g specific heat of water = 4.184 J/g°C specific heat of ice = 2.03 J/g°C heat of vaporization of water = 2260J/g
Answers
Answer:
\(Q=30298J\)
Explanation:
Hello,
In this case, in this heating process, we should consider two steps which have their own associated enthalpy for the same mass of water:
1) Melting of ice at 0 °C.
\(H_1=m_{ice}\Delta _{fusion}H=50g*334\frac{J}{g} =16700J\)
2) Heating of liquid water from 0 °C to 65 °C.
\(H_2=mCp(T_2-T_1)=50g*4.184\frac{J}{g\°C}(65-0)\°C =13598J\)
Therefore, the total needed heat turns out:
\(Q=H_1+H_2=16700J+13598J\\\\Q=30298J\)
Regards.
Answer:
30,298 joules of heat are needed to melt 50 g of ice at 0°C and then warm the liquid to 65°C
Explanation:
To analyze the heat that must be supplied to the ice, two phases are analyzed: one in which a part of heat will be required to melt the ice, that is, convert 0 ° C of ice to 0 ° C in a liquid state, and another part of heat that will raise the temperature of the melted ice to 65 ° C. So:
Total heat required = Heat required to melt ice + Heat required to raise the temperature of the ice in liquid state
Being fusion, the process that a substance undergoes to go from a solid state to a liquid, then the heat required to convert 0 ° C of ice to 0 ° C in a liquid state is calculated as:
Heat required to melt ice=mass*heat of fusion of ice= 50 g* 334 \(\frac{J}{g}\)= 16,700 J
On the other hand, the amount of heat received or transferred by a body when it undergoes a temperature variation (Δt) without there being a change of physical state (solid, liquid or gaseous) is calculated by the expression:
Q = c * m * ΔT
Where Q is the heat exchanged by a body of mass m, made up of a specific heat substance c and where ΔT is the temperature variation (Tfinal-Tinitial).
Then, the heat required to go from water at 0 ° C to water at 65 ° C is calculated by:
q=specific heat of water*m*ΔT= 4.184 \(\frac{J}{g*C}\) *50 g* (65 °C - 0°C)= 13,598 J
So:
Total heat required = 16,700 J + 13,598 J
Total heat required = 30,298 J
30,298 joules of heat are needed to melt 50 g of ice at 0°C and then warm the liquid to 65°C
Here is a more complex redox reaction involving the permanganate ion in acidic solution
Answers
In the redox reaction involving the permanganate ion in acidic solution, permanganate ion is reduced to manganese (ii) ion while iron (ii) ion is oxidized to iron(iii) ion.
What is a redox reaction?A redox reaction is a reaction in which reduction and oxidation occurs at the same time and to the same extent.
An example of a redox reaction is the reaction involving the permanganate ion and tetraoxosulphate (vi) ion in acidic solution.
The equation of the reaction is given below:
\(MnO_{4}^{ - } (aq) + 5Fe^{2+}(aq) + 8H^{+} \rightarrow Mn^{2+}(aq)+ 5Fe^{3+}(aq) +4H_{2}O(l) \\ \)
Therefore, in the redox reaction involving the permanganate ion in acidic solution, permanganate ion is reduced to manganese (ii) ion while iron (ii) ion is oxidized to iron(iii) ion.
Learn more about redox reaction at: https://brainly.com/question/26750732
#SPJ1
Of the bonds below, __________ is the least polar.
A) C,F
B) Na, Cl
C) Si, Cl
D) P,S
E) Na, S
Answers
P-S bond is the least polar.
Polarity is defined as the separation of charge between two atoms participating in a bond, giving rise to a dipole moment and thus a difference in electronegativity(tendency to pull electrons towards itself).
Different atoms have different values of electronegativity
The electronegativity values of the given elements are:
C= 2.55
F=3.98
Na=0.93
Cl=3.16
Si=1.9
P=2.19
S=2.58
Electronegativity difference between:
A) C,F = 3.98-2.55 =1.43
B) Na,Cl = 3.16-0.93=2.23
C) Si,Cl = 3.16-1.9=1.26
D) P,S= 2.58-2.19=0.39
E) Na,S= 2.58-0.93= 1.65
The electronegativity difference between P and S is the least, so P-S bond will be least polar.
To know more about polarity here
https://brainly.com/question/1578097
#SPJ4
is the longest stage of the cell cycle.
Answers
Answer:
Interphase is the longest part of the cell cycle, this is when the cell grows and copies its DNA before moving into mitosis.
Explanation:
Answer:
Interphase
Explanation:
How many revolutions does the hour hand on a clock make in a year using dimensional analysis for high school
Answers
a tire will burst if the air inside it reaches a pressure greater than 1.4 x 10^3 kpa. at what temperature will the tire burst if it has a volume of 30L and contains 2.5 mol of air? assume that the air behaves as an ideal gas. assuming that these values are representative, do you need to worry about your car tire bursting from overheating of they are in good condition?
Answers
This extremely high temperature indicates that under normal conditions, you do not need to worry about your car tire bursting from overheating as it is unlikely to reach such extreme temperatures.
To determine the temperature at which the tire will burst, we can use the ideal gas law equation:
PV = nRT
Where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
Rearranging the equation to solve for temperature, we have:
T = PV / (nR)
Given that the pressure threshold for bursting is 1.4 x 10^3 kPa, the volume is 30 L, and the number of moles of air is 2.5 mol, we can substitute these values along with the ideal gas constant R = 8.314 J/(mol K) into the equation.
T = (1.4 x 10^3 kPa) * (30 L) / (2.5 mol * 8.314 J/(mol K))
Converting kPa to Pa and L to m^3, and simplifying the equation, we find:
T ≈ 20,993 K
This extremely high temperature indicates that under normal conditions, you do not need to worry about your car tire bursting from overheating as it is unlikely to reach such extreme temperatures.
For more question on temperatures
https://brainly.com/question/4735135
#SPJ8
A mixture of 14.2 g of H2 and 36.7 g of Ar is placed in a 100.0 L container at
STP.
a. What is the total pressure in atmospheres inside the container?
b. What is the mole fraction and partial pressure of H2 in atmospheres?
c. What is the mole fraction and partial pressure of Ar in atmospheres?
Answers
a) The total pressure of the system is 1.79 atm
b) The mole fraction and partial pressure of hydrogen is 0.89 and 1.59 atm respectively
c) The mole fraction and the partial pressure of argon is 0.11 and 0.19 atm.
What is the total pressure?We know tat we can be able to obtain the total pressure in the system by the use of the ideal gas equation. We would have from the equation;
PV = nRT
P = pressure
V = volume
n = Number of moles
R = gas constant
T = temperature
Number of moles of hydrogen = 14.2 g/2g = 7.1 moles
Number of moles of Argon = 36.7 g/40 g/mol
= 0.92 moles
Total number of moles = 7.1 moles + 0.92 moles = 8.02 moles
Then;
P = nRT/V
P = 8.02 * 0.082 * 273/100
P = 1.79 atm
Mole fraction of hydrogen = 7.1/8.02 = 0.89
Partial pressure of hydrogen = 0.89 * 1.79 atm
= 1.59 atm
Mole fraction of argon = 0.92 / 8.02
= 0.11
Partial pressure of argon = 0.11 * 1.79 atm
= 0.19 atm
Learn more about partial pressure:https://brainly.com/question/13199169
#SPJ1
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
Imagine that scientists have just discovered a non-bird dinosaur skeleton. They want to know whether the dinosaur was closely related to birds. What features in ...
might help them decide?
Answers
Answer:
This evidence includes fossilized bones, teeth, eggs, footprints, teeth marks, and even dung. When paleontologists compare a skeleton of a living bird to the fossilized skeleton of a non-bird theropod, like Sinornithosaurus, they see many similarities.
Explanation:
Answer:
When people think of dinosaurs, two types generally come to mind. There were the huge herbivores,
like Apatosaurus, with their small heads and long tails. There were also those fearsome carnivores,
like Tyrannosaurus rex, that walked on two legs and had a mouthful of teeth like kitchen knives.
Living Dinosaurs
These large dinosaurs are no longer around, but dinosaurs still live among us today. They are the
birds. It's difficult to imagine that a bird on your window sill and a T. rex have anything in common.
One weighs less than a pound. The other was the size of a school bus, tipping the scales at eight
tons. But for all their differences, the two are more similar than you might think. In fact, birds and T.
rex are close relatives. They all belong to a group of dinosaurs called theropods.
This is a cladogram, a "" showing the relationships among organisms. The group called dinosaurs includes the extinct dinosaurs
and all their living descendants. All its members, including living birds, descended from the very first dinosaur-their common ancestor.
That's why birds are a kind of dinosaur (just as humans are a kind of primate).
Skeletal Evidence
When paleontologists compare a skeleton of a living bird to the
fossilized skeleton of a non-bird theropod, like Sinornithosaurus,
they see many similarities. They both have a hole in the hipbone, a
feature that distinguishes most dinosaurs from all other animals.
This feature allows an animal to stand erect, with its legs directly
beneath its body. All theropod dinosaurs, including birds, have a
furcula, also known as a wishbone. Another shared characteristic is the presence of hollow bones.
Hollow bones reduce the weight carried by an animal. This feature enables the animal to run faster. It
probably also played a role in the evolution of flight.
thought to have evolved for flight. The discovery of more and more non-flying dinosaurs with feathers
disproved that explanation. For these dinosaurs, feathers may have served other functions, like
gliding, insulation, protection, and display. Feathers play that same role in many bird species today.
Based on the evidence of shared characteristics, scientists have concluded that birds are a type of
Birds are the only dinosaurs with the ability to fly. This is
very interesting to scientists who want to know when the
capability of flight emerged. To find out, some scientists
study the brains of bird and non-bird dinosaurs. Soft
tissue, such as brains, is almost never preserved in the
fossil record. What is preserved is the imprint the brain
left on the inside of the skull. Now scientists are using
computed tomography (CT) scanners to create
endocasts. These are detailed, three-dimensional
reconstructions of the interiors of fossilized skulls.
In a recent study, researchers were able to peer inside
the braincases of more than two dozen specimens.
"Technology allows us to look inside these specimens
without destroying them," says Dr. Amy Balanoff, a
Museum research associate. "It's a non-destructive way
to basically slice up a dinosaur brain. We look inside and see what it can tell us about the evolution of
the brain within dinosaurs. Most of us grew up thinking that dinosaurs had tiny brains, but actually
some had really big brains."
The endocasts allow Balanoff and other researchers to
explore the outer shape of the brain in more detail. In
addition, the casts also provide new information about
the volume and shape of different regions of the brain.
For example, scientists looked at a detailed view of the
dinosaur cerebrum, a region of the brain related to
cognition and coordination. They found that this region
was very large in non-bird dinosaurs closely related to
birds. Dr. Balanoff's research suggests that these
dinosaurs developed big brains long before flight and that
these bigger brains prepared the way for them to fly.
When examining skeletal, behavioral, and brain
evidence, scientists see that birds and non-bird dinosaurs
share many features. This helped them conclude that
dinosaurs aren't extinct after all. They're living among us today.
(Im a really fast Typer and Thinker)
Have a nice day
why are divergent boundaries are also called a constructive boudary
Answers
Answer: Divergent boundaries are where two of those plates are moving away from each other. They are called constructive plates because when they move apart, magma rises up in the gap- this forms volcanoes and eventually new crust.
Explanation:
What are the three main groups of macronutrients? (select three please)
minerals
proteins
fats
carboydrates
Answers
Answer:
protein, carbs, and fats.
Explanation:
Those are the three main sources of macronutrients.
(this isn't a chemistry, it's health)
Answer:
fats, carbohydrates and proteins
Explanation:
I don't know, I answered that on my test and it was correct
Gold is yellow, shiny, smooth, and is found in the ground. A geologist finds a material that she thinks may be gold. Which of the following tests would reveal that
the material is not gold and not an element?
A. Its density is different from that of gold.
B. Its melting point is different from that of gold.
C. The material is composed of two different substances.
D. Its boiling point differs from that of gold.
Answers
Bleach is more basic than egg whites.
Bleach is more basic than egg whites.
Egg whites are more basic than bleach.
Egg whites are more basic than bleach.
Bleach is more acidic than egg whites.
Bleach is more acidic than egg whites.
Egg whites are more acidic than bleach.
Answers
An Amount of carbon – 12 Atoms equal to Avogadro‘s number would have a mass of exactly
A) 1 gram
B) 6 grams
C) 12 grams
D) 6.02 x 10 23 grams
Answers
Explanation:
the answer is C12 grams
How do you prepare 100mL of 0.5mL using your stock solution?
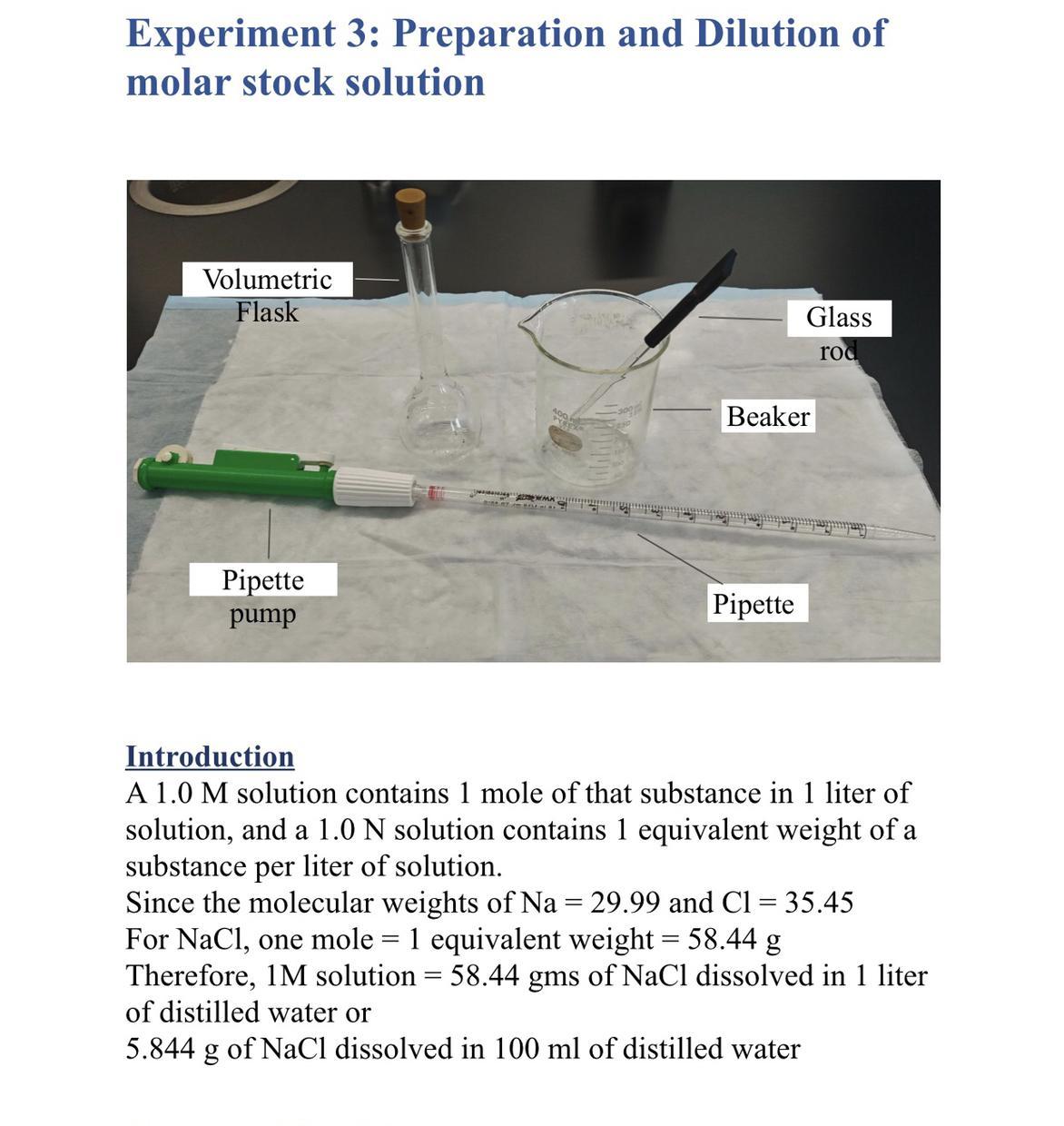
Answers
ANSWER
The concentration of the solution is 0.005M
EXPLANATION
Given that
The concentration of the stock solution is 1M
The volume of the stock solution to be diluted is 5mL
The final volume of the solution is 100mL
Follow the steps below to find the concentration of the resulting solution
Apply the dilution formula
C1 V1 = C2 V2
Substitute the given data into the above formula
\(\begin{gathered} 1\text{ }\times\text{ 5 = C2 }\times\text{ 100} \\ 0.\text{5 = 100C2} \\ \text{ Divide both sides by 100} \\ \text{ }\frac{\text{ 0.5}}{\text{ 100}}\text{ = C2} \\ \text{ C2 = 0.005M} \end{gathered}\)Therefore, the concentration of the solution is 0.005M
What is the formula of a hydrate that is 86.7% MoSs and 13.3% H20?
Answers
The formula of the hydrated compound based on the mole ratio is MoS.H₂O.
What are hydrated salts?Hydrated salts are salts that contain water molecules in a molecule of the compound.
In hydrated compounds, a molecule of the compound combines with a given number of moles of water.
The formula of the hydrate is calculated as follows:
Mass percentage of the anhydrous compound, MoS = 86.7%
Moles of MoS = 86.7% / 128
moles of MoS = 0.68
Mass percentage of water is 13.3%
Moles of water = 13.3 / 18
moles of water = 0.74
Mole ratio is 1 : 1
Formula of compound is MoS.H₂O
Learn more about hydrated compounds at: https://brainly.com/question/22666348
#SPJ1
How much copper (Cu) is in 4.64 g of copper?
Answers
answer
4.64 9 x 8 times
expo
How many grams of AuCl3 contain 5.0 x 1023 molecules?
Answers
Answer:
approximately 251.55 grams of AuCl3 would contain 5.0 x 10^23 molecules.
35. How many atoms are in 39.2 moles? (1 mole = 6.02 x 10 23 atoms)
A. 2.36 x 10 25atoms
B.
6.5 x 10 23 atoms
C.
6.5 x 10-23 atoms
D.
236 x 10 23 atoms
STOP
Answers
How many milligrams are in 2.89 x 1024 CO2 molecules?
Answers
Answer:
2,959.36
Explanation:
There are 2959.36 milligrams present in 2.89 x \(10^{24}\) CO2 molecules.
What is molecule?The smallest component of a substance which possesses both its chemical as well as physical characteristics. One or even more atoms make up molecules.
What is milligrams?Such a measure of mass as well as weight that weighs 0.0154 grains or one-thousandth of a gram.
Calculation of milligram in CO2 molecule.
Mass = No. of molecule / molar mass.
Molar mass = 44.02 g/mol.
Mass = 2.89 x \(10^{24}\) /\(6.023 * 10^{23}\)× 44.02 g/mol.
Mass = 2959.36 milligrams
To know more about molecule and milligram.
https://brainly.com/question/19922822
#SPJ3
The psychological response to aging is negatively influenced by what
Answers
Answer:
isolation, loneliness or psychological distress
Explanation:
In addition, older people are more likely to experience events such as bereavement, or a drop in socioeconomic status with retirement. Sadly these stressors can result in isolation, loneliness or psychological distress in older people. Mental health has an impact on physical health and vice versa.
1. What is the solubility of cerium sulfate at 10 degrees Celsius?
2. What is the solubility of cerium sulfate at 50 degrees Celsius?

Answers
Answer:
1. 10 g / 100 g H₂O
2. 2 g / 100 g H₂O
Explanation:
Both questions ask about the solubility of cerium sulfate (Ce₂(SO₄)₃), meaning we need to focus on the dark blue line.
1. When X = 10 °C, the value in the Y axis is 10. So the solubility of cerium sulfate at 10 °C is 10 g/100 g H₂O.2. Simmilarly, when x = 50 °C, Y is equal to 2 g / 100 g H₂OConsider the following reaction: 2N2O5(g) → 4NO2(g) + O2(g) Calculate the volume N2O5 that must decompose completely to produce 9.64 L nitrogen dioxide.
Answers
The volume of \(N_2O_5\) needed to produce 9.64 L of \(NO_2\) is 4.97 L, calculated using stoichiometry and the ideal gas equation.
The given chemical equation is \(2N_2O_5(g) \rightarrow 4NO_2(g) + O_2(g)\) .The volume of \(N_2O_5\) that decomposes completely to form 9.64 L of \(NO_2\) is to be calculated. For this, we can use the concept of stoichiometry. Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in a balanced chemical equation.To calculate the volume of \(N_2O_5\) that is needed to produce 9.64 L of \(NO_2\), we will first determine the number of moles of NO2 produced in the reaction. For this, we can use the ideal gas equation, PV = nRT. Here, we have the volume of NO2 and we can assume the pressure and temperature to be constant. Thus, we have PV = nRT, where P = pressure, V = volume, n = number of moles, R = ideal gas constant, and T = temperature. Substituting the given values in the ideal gas equation, we get,n = PV/RT = (1 atm × 9.64 L)/(0.0821 L atm K-1 mol-1 × 300 K) = 0.404 molFrom the chemical equation, we see that 2 moles of \(N_2O_5\) give 4 moles of \(NO_2\). Thus, 0.404 mol of \(NO_2\) must have been produced from (0.404/2) = 0.202 mol of \(N_2O_5\). Using the ideal gas equation, we can also find the volume of 0.202 mol of \(N_2O_5\) at the given conditions. Thus, V = nRT/P = (0.202 mol × 0.0821 L atm K-1 mol-1 × 300 K)/1 atm = 4.97 L. Thus, the volume of \(N_2O_5\) that must decompose completely to produce 9.64 L nitrogen dioxide is 4.97 L.For more questions on stoichiometry
https://brainly.com/question/14935523
#SPJ8
How does light interact with materials
Answers
Coal, oil, and natural gas are called _______.
A.
fossil fuels
B.
renewable resources
C.
nuclear power
D.
solar energy
Answers
Answer:
I think it's A. I'm pretty sure.
Explanation:
Answer:
Coal, oil and natural gas are called _________A. fossil fuels
Reaction type for this equation
2 AgNO3 + Cu → Cu(NO3)2 + 2 Ag
Answers
Answer:
single displacement reaction
The given chemical equation represents single displacement reaction as an more reactive element displaces less reactive element from it's compound.
What is chemical equation?
Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
The first chemical equation was put forth by Jean Beguin in 1615.By making use of chemical equations the direction of reaction ,state of reactants and products can be stated. In the chemical equations even the temperature to be maintained and catalyst can be mentioned.
Learn more about chemical equation,here:
https://brainly.com/question/28294176
#SPJ2
