Answers
Answer:
Linear
Explanation:
Related Questions
How many electrons are in Fe3+ ?
Answers
Answer:
There are 23 electrons in Fe3+
Science- I need help with a question really quick I’m ending school soon

Answers
What is the reaction??

Answers
HELP PLEASE I NEED HELP THANKS I LOVE U
How many moles of potassium nitrate (KNO3) are there in 0.300 L of a 2 molar solution?
Answers
Answer:
0.500-Molarity solution
Explanation:
The moles of the compound is given as the number of atomic mass unit in the compound. The moles of potassium nitrate in the solution are 0.6 mol
What is molarity?The molarity is the concentration unit, and it can be defined as the moles of compound present in the liter of solution.
The molarity can be expressed as:
\(\rm Molarity=\dfrac{Moles}{Volume(L)}\)
The given potassium nitrate solution has, molarity = 2 M
The volume of the solution is 0.3 L.
Substituting the values for the moles of the compound:
\(\rm 2\;M=\dfrac{Moles}{0.3\;L} \\\\Moles=2\;\times\;0.3\;mol\\Moles=0.6\;mol\)
The moles of potassium nitrate in 2 M solution is 0.6 mol.
Learn more about moles, here:
https://brainly.com/question/15209553
What volume, in liters, is occupied by 1.5 x 1023 atoms of argon gas (Ar) at STP?
Answers
ANSWER
The volume of Argon gas is 5.60L
STEP-BY-STEP EXPLANATION:
Given information:
The number of atoms of argon = 1.5 x 10^23 atoms
To find the volume of Argon, we need to find the number of moles of argon, then, we assume the number of moles of argon to be x
To find the number of moles, we will need to apply the below formula
\(\text{Number of atoms = mole x Avogadro's number}\)Recall that, Avogadro's number = 6.022 x 10^23
\(\begin{gathered} 1.5\cdot10^{23}\text{ = x }\cdot\text{ 6.022 }\cdot10^{23} \\ \text{Isolate x by dividing through by 6.022 }\cdot10^{23} \\ x\text{ = }\frac{1.5\cdot10^{23}}{6.022\cdot10^{23}} \\ x\text{ = 0.2498 mole} \end{gathered}\)Since x represents the number of moles of argon gas, then, the mole of argon gas is 0.2498 mole.
The next step is to find the volume of Argon in liters
Recall that, at S.T.P, 1 mole is equivalent to 22.4L
Let x represent the volume in liters of argon
Then, this can be solved mathematically below
\(\begin{gathered} 1\text{ mole }\rightarrow\text{ 22.4 L} \\ 0.2498\text{ mole }\rightarrow\text{ xL} \\ \text{Mathematically,} \\ 1\text{ = 22.4} \\ 0.2498\text{ = x} \\ \text{Cross multiply} \\ 1\cdot\text{ x = 0.2498 }\cdot\text{ 22.4} \\ x\text{ = }5.60L \end{gathered}\)Therefore, the volume of Argon gas is 5.60L
what is the formula for pentasulfur decaoxide
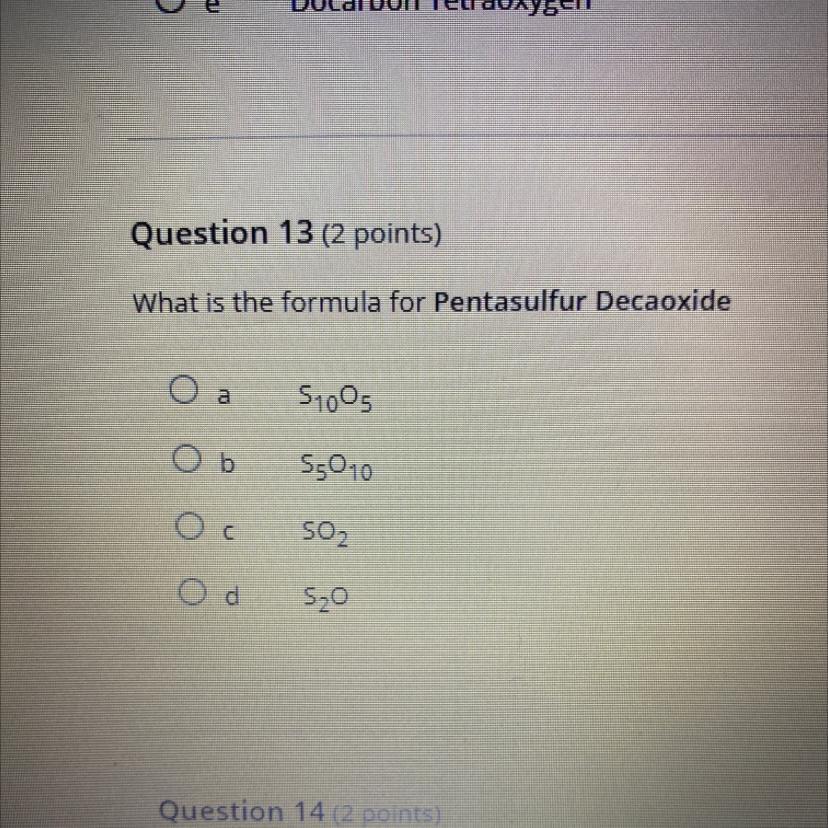
Answers
Answer:
S5O10 (b)
Explanation:
what causes the different crusts to rise and lower?
Answers
Answer:
tectonic plates
Explanation:
Cu+2AgNO 3 Cu(NO 3 ) 2 +2Ag what are the reactants

Answers
Answer:
Cu + 2AgNo3 is the reactants
A molecule that contains three identical polar bonds to the central atom will be?
Answers
The question is incomplete, the complete question is;
A molecule that contains three identical polar bonds to the central atom will be ________.
nonpolar if the geometry is planar triangular
polar in all cases
nonpolar in all cases
impossible to tell the polarity
either polar or nonpolar depending on the identity of the atoms bonded to the central atom
Answer:
Nonpolar if the geometry is planar triangular
Explanation:
The polarity of molecules depends both on the polarity of individual bonds in the molecule as well as the overall dipole moment of the molecule. We must remember that dipole moment is a vector quantity hence direction of the resultant vector is very important.
Now, if i have a molecule that contains three identical polar bonds, a planar triangular geometry means that the molecule is symmetrical and will have an overall dipole moment of zero. Hence the molecule is nonpolar.
How does an object become electrically charged?
A. Though the transfer of charges from one object to another
B. Through the movement of heat from one object to another
C. Through the transfer of sound from one object to another
D. Through the movement of water from one object to another
Answers
Answer: A
Explanation:
Charge cant be created or destroyed, it is only transferred between objects.
Answer:
And it's A
ok now make the first person brainiest
Explanation:
Hope this helps
Can someone please help me with this question. I got half of the question and I am stuck on the rest.

Answers
The mean of the data set is approximately 4.0626, and the 90% confidence interval is [4.060925, 4.064275].
What is the mean and 90% confidence interval of the given data?The sample mean (x) is calculated as follows:
x = (4.0620 + 4.0550 + 4.0650 + 4.0740 + 4.0550 + 4.0660) / 6
x ≈ 4.0626 (rounded to four decimal places)
The 90% confidence interval is calculated as follows;
Standard deviation (s):
(4.0620 - 4.0626)² = 0.00000036
(4.0550 - 4.0626)² = 0.00000576
(4.0650 - 4.0626)² = 0.00000006
(4.0740 - 4.0626)² = 0.00001328
(4.0550 - 4.0626)² = 0.00000576
(4.0660 - 4.0626)² = 0.00000012
average of the squared differences:
(0.00000036 + 0.00000576 + 0.00000006 + 0.00001328 + 0.00000576 + 0.00000012) / 6 ≈ 0.00000624
s = √(0.00000624)
s ≈ 0.002496
the standard error of the mean (SEM):
SEM = 0.002496 / √6
SEM ≈ 0.001018
For a 90% confidence interval, the z value is approximately 1.645.
ME = 1.645 * 0.001018 ≈ 0.001675
CI = x ± ME
CI = 4.0626 ± 0.001675
CI ≈ [4.060925, 4.064275]
Learn more about mean and confidence intervals at: https://brainly.com/question/20309162
#SPJ1
3. A graph of the cooling curve of a pure substance is plotted. When is the graph level with the time axis?(2)
A. Never
B.At the melting point only
C.At the boiling point only
D.At both the melting point and the boiling point
Answers
A substance's cooling curve is a graph representing the change in temperature over time as it is allowed to cool. The gradient of the cooling curve is influenced by the heat capacity, thermal conductivity, and ambient temperature of the material. The correct option is A.
The rate of cooling for a sample with a known composition is measured in order to map the phase boundaries on a phase diagram. As the sample (or some piece of it) starts to go through a phase change, the rate of cooling will alter. When the temperature-time curve changes slope, these "breaks" will be visible.
In the cooling curve, the graph never meet with the time axis.
Thus the correct option is A.
To know more about cooling curve, visit;
https://brainly.com/question/9680530
#SPJ1
3. How many grams of oxygen are required to completely react with 240g of C₂H6?
Answers
Approximately 766.08 grams of oxygen are required to completely react with 240g of C₂H₆.
To determine the amount of oxygen required to completely react with 240g of C₂H₆ (ethane), we need to set up a balanced chemical equation for the combustion of ethane.
The balanced equation for the combustion of ethane is as follows:
C₂H₆ + O₂ → CO₂ + H₂O
From the balanced equation, we can see that the stoichiometric ratio between C₂H₆ and O₂ is 1:3. This means that for every one mole of C₂H₆, three moles of O₂ are required for complete combustion.
To calculate the amount of oxygen required, we need to convert the given mass of C₂H₆ to moles using its molar mass, and then use the stoichiometric ratio to determine the moles of O₂ required. Finally, we can convert the moles of O₂ back to grams using the molar mass of oxygen.
The molar mass of C₂H₆ is calculated as follows:
(2 x atomic mass of carbon) + (6 x atomic mass of hydrogen)
(2 x 12.01 g/mol) + (6 x 1.01 g/mol) = 30.07 g/mol
Now, we can proceed with the calculation:
Calculate the moles of C₂H₆:
moles of C₂H₆ = mass of C₂H₆ / molar mass of C₂H₆
moles of C₂H₆ = 240 g / 30.07 g/mol ≈ 7.98 mol
Determine the moles of O₂ using the stoichiometric ratio:
moles of O₂ = moles of C₂H₆ x (3 moles of O₂ / 1 mole of C₂H₆)
moles of O₂ = 7.98 mol x 3 ≈ 23.94 mol
Convert moles of O₂ to grams:
mass of O₂ = moles of O₂ x molar mass of O₂
mass of O₂ = 23.94 mol x 32.00 g/mol = 766.08 g
For more such questions on oxygen visit:
https://brainly.com/question/28009615
#SPJ8
how to find moles, when given molar mass
Answers
To find moles when given the molar mass, you can use the concept of molar mass as a conversion factor.Molar mass represents the mass of one mole of a substance, expressed in grams per mole (g/mol).
To calculate moles, divide the given mass of the substance by its molar mass. The equation is:
moles = mass / molar mass
For example, if you have 56 grams of carbon dioxide (CO2) and want to find the number of moles, you need to know the molar mass of CO2, which is approximately 44 g/mol. Using the equation above:
moles = 56 g / 44 g/mol
moles ≈ 1.27 mol
Therefore, there are approximately 1.27 moles of carbon dioxide in 56 grams.
For more such questions on molar mass
https://brainly.com/question/29424807
#SPJ8
All isotopes of an element have a different number of ____.
a.
orbital shells
b.
neutrons
c.
protons
d.
electrons
e.
atoms
Answers
Protons and electrons have the same amount and atoms.
100 POINTS HELPPP Which of these is a potential use for a beaker?
A. Obtaining reagents from the original bottle
B. Holding a liquid that evaporates fast at room temperature
C. Creating a specific concentration of a solution
D. Making specific (precise) measurements
Answers
Answer:
B. Holding a liquid that evaporates fast at room temperature
Explanation:
Beakers are useful as a reaction container or to hold liquid or solid samples. They are also used to catch liquids from titrations and filtrates from filtering operations. Laboratory Burners are sources of heat. Burets are for addition of a precise volume of liquid.
B. Holding a liquid that evaporates fast at room temperature
Consider 55 mL of water (H2O) in a beaker and 55 mL of acetone |(CHs)2CO in an identical beaker under identical conditions Complete the sentences to explain the relationship between the vapor pressure of water and acetone. Match the words to the appropriate blanks in the sentences.
larger than
more quickly than
the same as
weaker
smaller than
stronger
more slowly than
at the same rate as
1. Water will evaporate______ acetone
2. The vapor pressure of acetone is__________ than that of water at the same temperature.
3. This is because the intermolecular forces between acetone molecules are ___________than those between water molecules.
Answers
Answer:
1. more slowly than
2. larger than
3. weaker
Explanation:
Acetone molecules are bonded by very weak intermolecular forces when compared to that of the hydrogen bond between water molecules. This makes it very easy for acetone molecules to vaporize easily into its gaseous state, much more faster than water molecules (since the acetone molecules need a lesser amount of energy to break these bonds). Also, the boiling point of liquid acetone is much lower than that of water, meaning that it has a higher vapor pressure than that of water.
30 example of redox reaction
Answers
1. Combustion of gasoline in a car engine
2. Rusting of iron
3. Photosynthesis in plants
4. Respiration in animals
5. Corrosion of metals
6. Bleaching of hair with hydrogen peroxide
7. Formation of ozone in the atmosphere
8. Electroplating of metals
9. Burning of wood
10. Reaction between bleach and ammonia
11. Reaction between copper and nitric acid
12. Reaction between iron and hydrochloric acid
13. Reaction between zinc and sulfuric acid
14. Reaction between magnesium and hydrochloric acid
15. Reaction between aluminum and hydrochloric acid
16. Reaction between sodium and water
17. Reaction between potassium and water
18. Reaction between lithium and water
19. Reaction between calcium and water
20. Reaction between barium and water
21. Reaction between copper and silver nitrate
22. Reaction between lead and silver nitrate
23. Reaction between zinc and copper sulfate
24. Reaction between iron and copper sulfate
25. Reaction between magnesium and copper sulfate
26. Reaction between aluminum and copper sulfate
27. Reaction between sodium and chlorine
28. Reaction between magnesium and chlorine
29. Reaction between aluminum and chlorine
30. Reaction between zinc and hydrochloric acid.
Part B Consider the following four molecules. Which of these satisfy the octet rule and which do not? Drag the appropriate items to their respective bins.
Answers
\(CS_{2}\) and \(CO_{3} ^{2-}\) adhere to the octet rule. The octet rule is a chemical rule of thumb based on the fact that atoms from the major group prefer to pair in this manner.
Each atom possesses eight electrons in its valence shell, giving it the same electrical configuration as a noble gas, according to the octet rule. \(PCl_{5}\) is not acceptable octet rule. \(CO_{3} ^{2-}\) will meet the requirement as it has two extra electrons, giving it a -2 charge. just follows the rules. Phosphorus pentachloride (\(PCl_{5}\)) and sulfur hexafluoride are two examples of chemicals that defy the octet rule (\(SF_{6}\)). Part B Consider the four molecules below. Which of these follows the octet rule and which does not? Drag the relevant objects to
learn more about octet rule here:
brainly.com/question/865531
#SPJ4
The complete question is:
Consider the following four molecules. Which of these satisfy the octet rule and which do not?
1) \(PCl_{5}\)
2) \(CS_{2}\)
3) \(SF_{6}\)
4) \(CO_{3} ^{2-}\)
1. A solution contains an unknown amount of dissolved magnesium. Addition of0.0877 mol of Na2CO3 causes complete precipitation of all of the magnesium.What mass of magnesium was dissolved in the solution?
Answers
Answer:
4.25 g of magnesium.
Explanation:
What is given?
moles of Na2CO3 = 0.0877 moles.
molar mass of Mg (magnesium) = 24.3 g/mol.
Step-by-step solution:
First, let's state the chemical equation between Mg (magnesium) and Na2CO3:
\(\text{2 Mg+Na}_2CO_3\rightarrow Mg_2CO_3+2Na.\)You can see that 1 mol of Na2CO3 reacts with 2 moles of Mg, so let's see how many moles of Mg are being produced by 0.0877 moles of Na2CO3:
\(0.0877\text{ moles Na}_2CO_3\cdot\frac{2\text{ moles Mg}}{1\text{ mol Na}_2CO_3}=0.175\text{ moles Mg.}\)And the final step is to convert from 0.175 moles of Mg to grams using its molar mass. The conversion will look like this:
\(0.175\text{ moles Mg}\cdot\frac{24.3\text{ g Mg}}{1\text{ mol Mg}}=4.25\text{ g Mg.}\)The answer is that there is 4.25 g of magnesium dissolved in the solution of 0.0877 moles of Na2CO.
An ideal gaseous reaction (which is a hypothetical gaseous reaction that conforms to the laws governing gas behavior) occurs at a constant pressure of 45.0 atm and releases 66.7 kJ of heat. Before the reaction, the volume of the system was 8.80 L . After the reaction, the volume of the system was 3.00 L . Calculate the total internal energy change, ΔE , in kilojoules.
Answers
The change in the total energy of the system is -93.1kJ.
What is the first law of thermodynamics?Let us note that the term thermodynamics has to do with the study of heat. Recall that heat is that which leads to the increase in the temperature of a body. From the first la of thermodynamics, we know that heat can neither be created nor destroyed but can be transformed from one form to another as we can see.
We also know from the first law of thermodynamics that;
ΔE = q + w
ΔE = total internal energy
q = heat absorbed or evolved
w = work done
Let us find the work done as;
w =pΔV
w = 45.0 atm (3 - 8.8)
w = -261 atm L or -26.4 kJ
We have q as -66.7 kJ because heat was released
ΔE = -66.7 kJ - 26.4 kJ
ΔE = -93.1kJ
Learn more about thermodynamics:https://brainly.com/question/1368306
#SPJ1
Steel is an alloy of iron and carbon. The addition of carbon to iron enhances which of the following properties of iron metal?
O A hardness
O B. malleability
O C. ductility
O D. softness
Answers
Answer:
The answer should be A. Adding carbon to iron makes it tougher and stronger.
The addition of carbon atom to iron metal, enhances the property of hardness of metal.
What is alloy?Alloy is a compound which is formed by the mixture of two or more than two metals with different properties to make a new compound with better properties.
When we add carbon atom in the iron metal, it deviates the crystal lattice property of iron and makes it more harder. So, the content of carbon in the iron is directly proportional to the hardness of iron metal.
Hence, option (A) is correct i.e. hardness.
To know more about hardness, visit the below link:
https://brainly.com/question/18173707
Which is an aspect of the kinetic-molecular theory and can be used to explain the behavior of plasmas? A. Particle spacing can allow a very high density. B.Particle kinetic energy is independent of temperature. C. Particles vibrate quickly in stationary positions. D. Particles exchange energy through elastic collisions.
Answers
Answer:
D: Particles exchange energy through elastic collisions.
Explanation:
took a test
The aspect of the kinetic-molecular theory and can be used to explain the behavior of plasmas is " Particles exchange energy through elastic collisions."
What is kinetic-molecular theory ?The assumption that matter being made up of microscopic particles which is always in motion forms the foundation of the kinetic-molecular theory, which describes the states of matter. This theory explains the characteristics and behaviors of gases, liquids, as well as solids that can be observed.
What is elastic collisions?
A collision among both two bodies that is elastic occurs when their combined kinetic energy stays constant.
The aspect of the kinetic-molecular theory and can be used to explain the behavior of plasmas is " Particles exchange energy through elastic collisions."
To know more about elastic collision and kinetic molecular theory.
https://brainly.com/question/15013597
#SPJ2
I need help on this Chem problem

Answers
Four Hydrogen atoms are present in the molecule (Furan) shown below.
Furan is a heterocyclic organic compound with a five-membered ring containing four carbon atoms and one oxygen atom. It has the chemical formula C4H4O. Furan is a colorless, volatile liquid with a distinctive aromatic odor. Coal tar and organic material burning make it.
Furan is utilised in resins, polymers, and solvents. Furan is poisonous and carcinogenic, hence its use and exposure must be carefully controlled.
Learn more about Furan, here:
https://brainly.com/question/29753641
#SPJ1
What happens to the amount of solution when we add food colour to it?
Answers
Answer:
We need more? What else is in the question? This is unanswerable.
Explanation:
Balancing chemical reactions, what is it and two examples!!
PLEASE HELP I NEED THE ANSWER!!!
Giving lots of POINTS
Answers
Answer:
A balanced chemical reaction is an equation where the number of atoms of each type in the response is the same on both reactants and product sides.
Explanation: Burning, and cooking are two examples. :)
Hope you are happy :)
ASAP PLZ HELP I WILL MARK BRAINLIEST Based on the diagram, what can you conclude about the pole of the magnet? O It is a south pole because the field lines spread out from this end. O It is a north pole because the field lines spread out from this end, O It is a south pole because the field lines enter the magnet at this end. O It is a north pole because the field lines enter the magnet at this end.

Answers
Answer:C
Explanation:I took the test
Answer:
It is a south pole because the field lines enter the magnet at this end.
Explanation:
In an isolated system, two copper bars at different temperatures transfer energy until both are at the same temperature. How would the transfer of
energy be different if the bars were in an open system?
OA Energy transfer would occur only between the copper bars.
OB. Energy transfer would occur between the copper bars and the surroundings.
OC. No energy transfer would occur between the copper bars or the surroundings.
OD. Energy transfer would occur only with the surroundings.
Answers
The manner in which the transfer of energy would be different if the bars were in an open system is as follows: Energy transfer would occur between the copper bars and the surroundings (option B).
What is law of conservation of energy?The law of conservation of energy principle stating that energy may not be created or destroyed.
An isolated system exchanges neither energy nor matter with the surroundings. According to this question, two copper bars at different temperatures transfer energy until both are at the same temperature.
However, in an open system, some of the energy would be transferred to the surroundings.
Learn more about energy transfer at: https://brainly.com/question/13087586
#SPJ1
Indicate the changes (increases, decreases, does not change) in its volume when the pressure undergoes the following changes at constant temperature and amount of gas. Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer. ResetHelp 1. The pressure increases to 6.0 atm. The volume The pressure increases to 6.0 a t m. The volume blank.. 2. The pressure drops to 0.40 atm. The volume The pressure drops to 0.40 a t m. The volume blank.. 3. The pressure remains at 2.0 atm. The volume The pressure remains at 2.0 a t m. The volume blank..
Answers
The question is missing information. Here is the complete question.
A gas at a pressure of 2.0 atm is in a closed container. Indicate the changes (if any) in its volume when the pressure undergoes the following changes at constant temperature and constant amount of gas. Match the words in the left with the column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer.
1. The pressure increases to 6.0 atm. The volume ________
2. The pressure drops to 0.40 atm. The volume _________
3. The pressure remains at 2.0 atm. The volume _________
Answer: 1. Decreases
2. Increases
3. Does not change
Explanation: According to the Ideal Gas Law, Pressure, Volume and Temperature of an ideal gas is related, as the following: PV = nRT.
In this case, since temperature (T) and amount of gas (n) are constant, the Boyle's Law can be used.
The law states that the volume of a given gas, under the conditios of temperature and amount of it are constant, is inversely proportional to the applied pressure: P₁.V₁ = P₂.V₂
For case 1.)Initial P (P₁) = 2
Initial V (V₁) = V
Final P (P₂) = 6
P₁.V₁ = P₂.V₂
2.V = 6.V₂
V₂ = 1/3V
When the pressure increases to 6 atm, volume decreases by 1/3.
For case 2.)P₁ = 2
V₁ = V
P₂ = 0.4
2.V = 0.4V₂
V₂ = 5V
When pressure drops to 0.4 atm, volume increases by 5.
For case 3.)Since there are no change in the pressure, the volume is the same from the beginning, so does not change.
4K + O2 + 2K20
Which statement is true?
Answers
Answer:
yes 4K + O2 ------>2K20 is true.
