how to check if the rain is acidic
Answers
Answer:
By collecting the rainwater carefully without allowing for any contamination one can then determine by an instrument known as a pH meter the acidity of the solution.
Explanation:
Related Questions
29.
How many seconds would it take for the total pressure to be 0.7133 atm?
Answers
4. 100 mL of an NaOH solution is neutralized by 50 mL of a 0.5M HCl solution. What is the molarity of the NaOH solution?
Please help with real answer
Answers
Answer:
6.098M
Explanation:
akording to m1v1/m2v2=n1/n2
what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1
Which part of modern atomic theory was first developed by John Dalton?
A. Atoms cannot be divided.
B. Electrons are spread throughout an atom.
O C. Atoms are rearranged during chemical reactions.
O D. Electrons are found in a cloud around the nucleus.
Answers
Answer:
C. Atoms are arranged during chemical reactions
Explanation:
All matter is made of atoms and atoms are indivisible and indestructible. compounds are formed by a combination of two or more different kinds of atoms.
An unopened, cold 2.00 L bottle of soda contains 46.0 mL of gas confined at a pressure of 1.30 atm at a temperature of 5.0 ℃. If the bottle is dropped into a lake and sinks to a depth at which the pressure is 1.52 atm and the temperature is 2.09 ℃, what will be the volume of the gas in the bottle? Explanation needed
Answers
Answer:
\(V_2= 38.9mL\)
Explanation:
Hello there!
In this case, since this is a problem about the combined gas law because the temperature, volume and pressure undergo a change:
\(\frac{P_2V_2}{T_2}= \frac{P_1V_1}{T_1}\)
Thus, since we need the final volume, V2, we solve for it as shown below:
\(V_2= \frac{P_1V_1T_2}{T_1P_2}\)
Now, we plug in the data to obtain:
\(V_2= \frac{1.30atm*46.0mL*(2.09+273)K}{(5+273)K*1.52atm}\\\\V_2= 38.9mL\)
Best regards!
A first order reaction has a half - life of 36 min. What is the value of the rate constant? A. 3.2 x 10-4 s-1. B. 1.9 x 10-3 L mol-1 s-1. C. 1.2 s-1
Answers
To determine the rate constant of a first-order reaction, we can use the equation for the half-life of a first-order reaction: t1/2 = ln(2) / k
Given:
Half-life (t1/2) = 36 min We need to convert the half-life from minutes to seconds to match the units of the rate constant. Therefore, t1/2 = 36 min * 60 s/min = 2160 s.
Now we can rearrange the equation and solve for the rate constant (k):
k = ln(2) / t1/2
Substituting the given value, we have:
k = ln(2) / 2160 s Calculating this expression, we find that the rate constant is approximately 3.214 x 10^(-4) s^(-1). Therefore, the correct answer is option A. 3.2 x 10^(-4) s^(-1).
Learn more about first-order reaction here: brainly.com/question/28213237
#SPJ11
How many moles 30.56g of Ca(OH)
Answers
Answer:
0.412 moles
Explanation:
No of moles =given mass /molar mass
30.56/74=0.412 moles
Note these are the number of moles of
Ca(OH)2 not Ca(OH)
a solution is prepared by mixing 694.0 ml of ethanol with 506.0 ml of water. the molarity of ethanol in the resulting solution is 10.32 m. the density of ethanol at this temperature is 0.7893 g/ml. calculate the difference in volume between the total volume of water and ethanol that were mixed to prepare the solution and the actual volume of the solution.
Answers
The difference in volume is -59.1 mL, indicating that the actual volume of the solution is slightly less than the initial volume of the water and ethanol that were mixed.
These are the following steps for calculation :-
First, we need to calculate the amount of ethanol in the solution:
moles of ethanol = molarity × volume of solution
moles of ethanol = 10.32 mol/L × 0.6940 L
moles of ethanol = 7.15488 mol
Next, we can calculate the mass of ethanol in the solution:
mass of ethanol = volume of ethanol × density of ethanol
mass of ethanol = 0.6940 L × 0.7893 g/mL
mass of ethanol = 0.547 grams
Now we can calculate the mass of water in the solution:
mass of water = volume of water × density of water
mass of water = 0.5060 L × 1 g/mL
mass of water = 0.5060 grams
The total mass of the solution is:
total mass = mass of ethanol + mass of water
total mass = 0.547 grams + 0.5060 grams
total mass = 1.053 grams
The density of the solution is:
density = total mass / total volume
total volume = total mass / density
total volume = 1.053 grams / (694.0 mL + 506.0 mL)
total volume = 1.053 grams / 1200 mL
total volume = 0.8775 g/mL
The difference in volume between the total volume of water and ethanol that were mixed to prepare the solution and the actual volume of the solution is:
difference in volume = actual volume - initial volume
difference in volume = 1 / 0.8775 - (694.0 mL + 506.0 mL)
difference in volume = 1.1409 L - 1200 mL
difference in volume = -59.1 mL
Therefore, the difference in volume is -59.1 mL, indicating that the actual volume of the solution is slightly less than the initial volume of the water and ethanol that were mixed.
To know more about volume visit :-
https://brainly.com/question/463363
#SPJ1
Select all that apply. when the products of a reaction have a lower enthalpy than the reactants _____. the reaction is endothermic. the reaction is exothermic. the change in enthalpy is positive. the reactants lost internal energy.
Answers
when the products of a reaction have a lower enthalpy than the reactants, the reaction is endothermic and the change in enthalpy is positive.
In Endothermic Reactions, heat is absorbed from the surroundings. This energy is required for the breakage of bonds so that the reaction can proceed. The overall temperature of the surroundings is decreased.
When heat is absorbed in a system the system has higher enthalpy at the end of the reaction. The change in enthalpy is positive when the reaction is endothermic.
Dissolution of ammonium chloride into water is an example of endothermic reactions.
If you need to learn more about endothermic reactions, click here
https://brainly.com/question/23184814?referrer=searchResults
#SPJ4
Answer: THE ANSWER BEFORE/AFTER MINE IS INCORRECT!!
The reaction is exothermic & The reactants lost internal energy.
I just got this one correct.
Hope I helped<3
Would you expect higher or lower conductivity from a solute that does not dissolve well in solution?
Answers
It will be lower conductivity from a solute that does not dissolve well in solution.
The conductivity of the solution will be lower than that of entirely dissociating compounds if an ionic compound does not completely dissociate.Ions are created when an ionic substance dissociates in water and dissolves. As is well knowledge, the degree of an ionic compound's dissociation directly relates to the conductivity of an ionic solution. An ionic compound's conductivity will be high if it dissociates more.The compound's conductivity will be lower if it dissociates less.The ionic compound with less dissociation would thus have lower conductivity, as may be deduced.To know more about conductivity visit : https://brainly.com/question/1336689
#SPJ1
Help me with these two please & thank you !

Answers
The heat of the reaction is 90.83 kJ/mol.
The specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius.
It is a measure of how much energy it takes to raise the temperature of a substance. It is the amount of heat necessary to raise one mass unit of that substance by one temperature unit.
It is given by the formula -
Q = mcΔT
where, Q = amount of heat
m = mass
c = specific heat
ΔT = Change in temperature
Heat of formation is the opposite of heat of reaction.
Learn more about Specific heat, here:
https://brainly.com/question/31608647
#SPJ1
How many grams of NaCl are in 1.25 X 1024
molecules of Naci? Use one decimal place
Answers
Answer:
121.1g
Explanation:
First, we need to convert the number of molecules of NaCl to moles (n) by dividing by Avagadro number
That is, n = number of molecules ÷ 6.02 × 10²³
n = 1.25 X 10²⁴ ÷ 6.02 × 10²³
n = 1.25/6.02 × 10^ (24-23)
n = 0.207 × 10¹
n = 2.07moles
Using the formula; mole = mass ÷ molar mass
Molar mass of NaCl = 23 + 35.5 = 58.5g/mol
mass = molar mass × mole
mass = 58.5 × 2.07
mass = 121.1g
need answer asap 4 both

Answers
The persistence of vision is regarded as an optical illusion but it is actually part of human physiology.
What is persistence of vision?The term persistence of vision is the idea that an object is able to remain is sight for a long time even when the object has been removed. This is often used by performers to create a remarkable impression on the audience. The persistence of vision is regarded as an optical illusion but it is actually part of human physiology.
The way that a performance can be designed to make the audience to know that this is the most important scene is called follow through.
Learn more about optical illusion:https://brainly.com/question/28179807
#SPJ1
Write each chemical bond or elements of the chemical formula:2Mg + O2 -> 2MgO As a Lewis dot structure (while still in the formatting of the chemical formula).
Answers
1) Chemical formula
\(2Mg+O_2\rightarrow2MgO\)2) Mg Lewis structure
3) O2 Lewis structure
4) MgO Lewis structure
5) Chemical formula written as Lewis dot structure
.




I need help 7th grade science

Answers
Answer:
a flowing stream
Explanation:
.........
the labs in this chemistry class use a green approach. what does that mean
Answers
The labs in this chemistry class use a green approach, which means they prioritize environmentally friendly practices.
In this chemistry class, the term "green approach" refers to a set of practices and principles that prioritize environmental sustainability and minimize negative impacts on the ecosystem. These labs aim to reduce their carbon footprint, conserve resources, and promote responsible waste management. By adopting a green approach, the class strives to align its scientific pursuits with the goal of environmental stewardship.
One of the key aspects of the green approach in these chemistry labs is the conscious selection and utilization of environmentally friendly materials and chemicals. This includes opting for safer alternatives to hazardous substances whenever possible, such as using non-toxic solvents or reagents. Additionally, the labs may encourage the use of renewable resources and promote the recycling or repurposing of materials to reduce waste generation.
Another important component of the green approach is energy conservation. The labs may employ energy-efficient equipment and lighting systems, as well as implement strategies to minimize energy consumption during experiments. For instance, they may encourage students to turn off equipment when not in use and adopt efficient heating or cooling methods.
Furthermore, the labs may focus on water conservation by promoting responsible water usage and minimizing water wastage during experiments. This could involve using water-efficient techniques, such as microscale experiments that require smaller amounts of water, or implementing recycling systems to capture and reuse water when appropriate.
By embracing a green approach, these chemistry labs aim to instill environmental awareness and responsibility in students while demonstrating that scientific progress can coexist with sustainable practices. Through this approach, students gain valuable knowledge and skills that they can apply in their future scientific endeavors, contributing to a more sustainable and eco-friendly society.
Learn more about green approach
https://brainly.com/question/3215987
#SPJ11
Which toxic substance is often used to extract gold and results in harmful environmental effects?
a. acid mine drainage
b. carbon dioxide
c. sulfur dioxide
d. cyanide
e. fluoride
Answers
Please help - Alevel questions on chapter 6 kinetics a level chemistry

Answers
(a) Within a reversible reaction, forward and reverse chemical reactions progress at the same pace while concentrations of products and reactants remain constant with time.
(b) Le Chatelier's principle states that an increase in pressure advantages a side containing fewer moles of gas. For instance, in the formation of methanol from CO and H2, one molecule of gas is formed while two molecules come from the elements themselves.
How to explain the reaction(c) Sustaining a pressure above 5 MPa would be costly as high-tensile materials, equipment and additional energy are needed to compress the gases at those pressures.
(d) Rising temperature allows for an equilibrium towards the endothermic direction, where heat consumption occurs. Here, since the exothermic reaction itself releases heat; it will result in a shift towards the reactant side, causing a fall in the yield rate of methanol produced.
(e) If a catalyst does not exist, reaction rates would slow down considerably, requiring higher temperatures to prompt reasonable reaction progression. This is because catalysts lower activation energy, enabling the reaction to be done at lesser temperatures.
Learn more about reaction on
https://brainly.com/question/11231920
#SPJ1
what is the most dangerous element to a star?
hydrogen
lithium
helium
iron
Answers
HELP PLEASE FAST 100 POINTS

Answers
In any chemical reaction, the mass of the products must equal the mass of the reactants. The mass of product B is the same as the combined mass of Reactant A and H₂O.
Answer:
sh5barch shougi
Explanation:
_____ is said to occur when a new product line reduces the sales of an existing line.
Answers
Cannibalization is said to occur when a new product line reduces the sales of an existing line.
Cannibalization happens when a company introduces a new product or service that competes with its existing product or service. This can lead to a decrease in sales of the existing product or service, as customers may choose to purchase the newer or more attractive offering.
While cannibalization can be detrimental to a company's bottom line in the short term, it may be necessary to remain competitive in the long term. Companies must carefully consider the potential impacts of introducing a new product or service on their existing product lines and overall market position. Proper planning and market research can help mitigate the negative effects of cannibalization and maximize the benefits of introducing new offerings.
To know more about the Product line, here
https://brainly.com/question/29488604
#SPJ4
A person with a mass of 105 kg moves at a velocity of 1.5 m/s what is this person momentum?
Answers
Answer:
225kg.m.s
Explanation:
Momentum equals to mass times velocity
Which of the following is a propagation step in the free radical chlorination of methane?
∙CH3 + Cl2 → CH3Cl + Cl∙
∙CH3 + Cl∙ → CH3Cl
∙CH3 + ∙CH3 → CH3CH3
Cl2 → ∙Cl + ∙Cl
Answers
The propagation step in the free radical chlorination of methane is:
∙CH₃ + Cl∙ → CH₃Cl
In the free radical chlorination of methane, the propagation step is a crucial part of the overall reaction mechanism. It involves the interaction between a methyl radical (∙CH₃) and a chlorine radical (Cl∙), resulting in the formation of chloromethane (CH₃Cl).
During the propagation step, the methyl radical (∙CH₃) and chlorine radical (Cl∙) combine to produce chloromethane (CH₃Cl). This reaction occurs through the abstraction of a hydrogen atom from methane by the chlorine radical, forming a new C-Cl bond and generating a new methyl radical. The overall reaction can be represented as follows:
∙CH₃ + Cl∙ → CH₃Cl
Learn more about chlorine and methane from the link given below.
https://brainly.com/question/2409985
#SPJ4
what is the number of moles of co2 in a 220 gram sample of co2
Answers
The number of moles of CO2 in a 220 gram sample can be calculated by dividing the mass of the sample by the molar mass of CO2.
The molar mass of CO2 (carbon dioxide) is calculated by adding the atomic masses of carbon (C) and oxygen (O) in one mole of CO2. The atomic mass of carbon is approximately 12.01 grams/mol, and the atomic mass of oxygen is approximately 16.00 grams/mol (rounded values).
Therefore, the molar mass of CO2 is approximately 12.01 + (16.00 x 2) = 44.01 grams/mol. To determine the number of moles of CO2 in a 220 gram sample, divide the mass of the sample (220 grams) by the molar mass of CO2 (44.01 grams/mol). The result will be the number of moles of CO2 in the given sample.
To learn more about molar mass click here :
brainly.com/question/30640134
#SPJ11
calculating the heat of reaction from molar reaction enthalpy and the mass of a reactant
Answers
1) Because ΔH is positive, the reaction is endothermic.
2) Yes, absorbed, because in endothermic reaction heat is absorbed.
3) Heat will be released 26.9 KJ
Enthalpy definitionEnthalpy depends only on the system's composition, temperature, and pressure; it is unaffected by the system's history. It is a quality or state function that resembles energy and has energy-like properties (and is thus measured in units of joules or ergs).
Given reaction is
2HgO(s) → 2Hg(l) + O₂(g) ΔH = 182KJ
1) Because ΔH is positive, the reaction is endothermic.
2) Yes, because heat is absorbed during endothermic reactions.
3) Heat will be released 26.9 KJ
According to the reaction,
2 moles HgO of release = 182 KJ heat
2×216.59 HgO release = 182 KJ heat
64 g HgO release = 182×64/2×216.59 KJ heat
64 g HgO release = 26.9 KJ.
To know more about enthalpy visit:
https://brainly.com/question/13996238
#SPJ4
Complete question is attached below

1) 5 small and 4 large cups of coffee cost $22.
2 small and 1 large cups of coffee cost $7.
58 + 4( ) = 22
2(58 + 4L = 22)
5(28 + 1L = 7)
Small
coffee =
Large
coffee =
large = ?
$2
22) → →
O
O
$3
O
O
$4
O
small = ??
Small coffees cost $
Large coffees cost $
$5 $6
O
O
O
O
2 points
$7
O
O
Answers
For the following balanced equation, which has the highest coefficient?
4 H2 + 2 C → 2 CH4
Answers
H2 has the highest coefficient.
Considering a balanced equation,
4 H2+2 C
————>2 CH4
=> The hydrogen molecule's (H2) coefficient is 4.
=> The carbon (C) coefficient is 2.
=> Methane CH4 has a coefficient of 2.
As a result, H2 has the greatest coefficient of 4, which is.
H2 has the highest coefficient, hence.
Gases made of hydrogen have no color or smell. It is quickly set ablaze. It burns with a light blue, nearly undetectable flame once lit. They are lighter than air, the vapors. It ignites easily at a variety of vapor/air concentrations. While asphyxiating easily due to the displacement of oxygen in the air, hydrogen is not poisonous.
To know more about hydrogen, visit;
https://brainly.com/question/28937951
#SPJ4
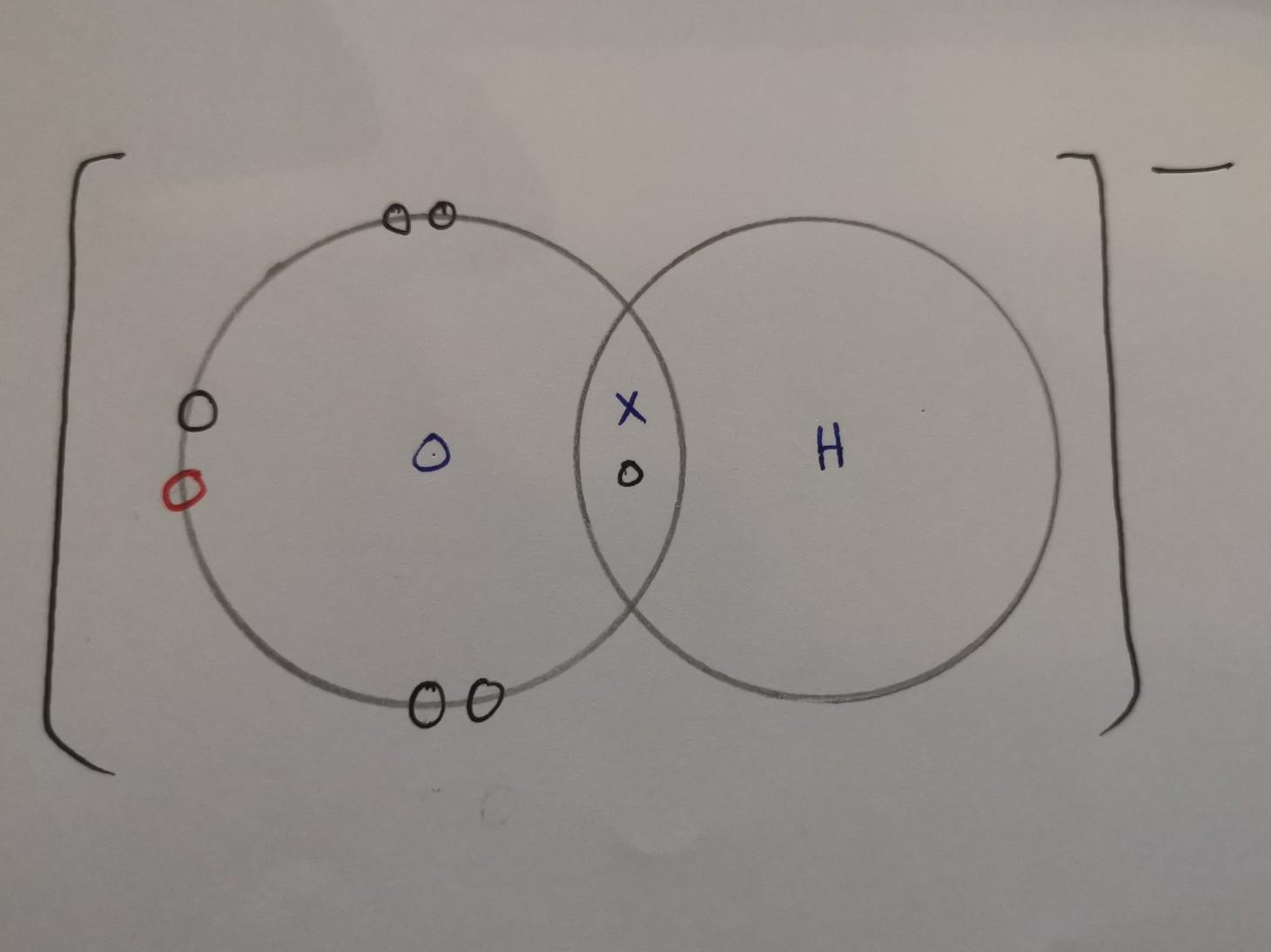
Draw the electron dot structure of the hydroxide ion (OH-).
Answers
Answer:
X = electrons from hydrogen
O (black) = electrons from oxygen
O (red) = electrons from when it was connected to a metal atom, hence why it has a negative charge
When something is in motion it is changing the location
mass
both location and mass
neither location or mass
Answers
I hope this helps! Please consider giving me the brainliest as i do put a lot of time and effort into each answer. Have a wonderful day!
this is probably almost the easiest question ever but i still need help so-

Answers
Soil is our life support system. Soils provide anchorage for roots, hold water and nutrients. Soils are home to myriad micro-organisms that fix nitrogen and decompose organic matter, and armies of microscopic animals as well as earthworms and termites.
bbbb