Answers
Related Questions
WORTH 100 POINTS, ANSWER ALL PARTS
INFO. An unknown compound containing tellurium and bromine is analyzed and it is determined that 28.53% of the compound by mass is composed of tellurium.
Part A. What quantity in moles of Te are present in 100.00 g of the compound?
Part B. What is the mass percent of Br in the compound?
Part C. What quantity in moles of Br are present in 100.00 g of the compound?
Part D. Determine the empirical formula of the unknown compound.
Part E. Provide the correct IUPAC name of the unknown compound.
Answers
PART B - 71.47% Br
PART C - 0.8945 mol Br
PART D - TeBr4
PART E - tellurium tetrabromide
Belching (burping) cows give off much of the methane, a greenhouse gas, from raising farm animals. Now researchers are investigating a way to reduce the greenhouse gas that is burped by cows.
In one study, scientists fed a group of dairy calves (which are cows that produce milk) a methane-blocker chemical. Sensors at feeding stations measured methane in the cows’ gas while the animals were nearby. That allowed the team of researchers to estimate the methane being belched (burped) out each day.
Over their first year of life, the treated cows, which received the methane-blocker chemical, released about ten percent less methane than the group of cows not given the chemical.
What can you infer or conclude based on the evidence from the passage?
*Your response should include the evidence to support your conclusion.
Answers
Cows and other ruminants are significant producers of the greenhouse gas methane—contributing 37 percent of the methane emissions resulting from human activity.
What is greenhouse gas?Greenhouse gases (also known as GHGs) are gases in the earth's atmosphere that trap heat. During the day, the sun shines through the atmosphere, warming the earth's surface. At night, earth's surface cools, releasing heat back into the air. But some of the heat is trapped by the greenhouse gases in the atmosphere.
While carbon dioxide is typically painted as the bad boy of greenhouse gases, methane is roughly 30 times more potent as a heat-trapping gas. As temperatures rise, the relative increase of methane emissions will outpace that of carbon dioxide from these sources, the researchers report.
The cow's ramen is like a large fermentation vat. More than 200 different bacteria and 20 types of protozoa help the cow to utilize fibrous feed stuffs and non-protein nitrogen sources. Bacteria adhere to the feed and gradually digest the fermentable material.“You can probably reduce methane by about 20-25% by altering diet,” he says.
One study by researchers at the University of California, Davis, estimated it might be possible to reduce global methane emissions from cows by 15% by changing their diet.
Thus, this evidence support the passage.
To learn more about greenhouse gas, refer to below link:
https://brainly.com/question/4509458
#SPJ2
how are digital road maps different from paper and rod map?
a. they allow users to plan routes before a trip
b. they can be used anywhere in the world
c. they can be updated almost immediately
d. they show major and minor roads in a region
please helppppp need it asap
Answers
Digital road maps are different from paper and road maps in that they allow users to plan routes before a trip, they can be used anywhere in the world, and they can be updated almost immediately. They also show both major and minor roads in a region.
Digital road maps are a type of digital map that is based on GIS data. This data is collected from various sources and is used to create a detailed representation of the real world. The digital road map allows the user to zoom in and out and to see different levels of detail, depending on their needs. They also allow the user to search for specific locations, find directions, and plan routes before they begin their trip.
On the other hand, paper maps are usually printed on paper and can be difficult to read in low light conditions. They are also limited in the amount of detail that they can show, and they may not always be up-to-date.
Road maps, on the other hand, are a type of map that shows roads and highways in a region. They may include some additional features such as rest areas, gas stations, and other points of interest. Road maps are typically printed on paper and are often used for navigation while driving. They are not as detailed as digital road maps, and they can quickly become outdated as new roads are built.
For more such questions on Digital road maps
https://brainly.com/question/12456226
#SPJ8
2 examples of metal’s catalytic reaction
Answers
Answer:
Example 1
palladium(II) nitrate,
Example 2
Metal catalysts such as Fe, Ni, Mo, and Co are routinely used in the manufacture of CNMs.
Explanation
The three metals used in catalytic converters — rhodium, platinum and palladium — are part of a category known as platinum group metals, or PGMs, which are known for their catalytic properties.
A company produces and tests a new mineral supplement designed to prevent the common
cold. The company tests three dosages of the mineral supplement on 300 participants, male
and female, between the ages of 25 and 50 over a 6-month period. Each group has 100
randomly assigned participants. The table shows the dosages of the mineral supplement given
to each participant in each group and the numbers of participants that report having the
common cold during the 6-month period.
The company claims that the mineral supplement can effectively prevent the common cold. A
scientist disputes the claim, saying an error exists in the setup of the groups for the
investigation.
Which change to the investigation would remove the source of this error?
Answers
A change which would remove the source of this error is to: Test a fourth group, in which participants do not receive the mineral supplements.
What is a scientific method?A scientific method can be defined as the various techniques that are adopted to observe, investigate, examine and study a particular thing while giving explanations on the basis of the evidence, theory, proof, hypothesis, or factual information they derived from the research work.
In this scenario, a claim was made by the company its mineral supplements can effectively prevent common cold. However, this claim was disputed by a scientist because there exist an error in the group setup.
How to remove an error in scienceFor this investigation, an effective and efficient way to remove this source of error would be by testing a fourth group, in which the mineral supplements is not administered to any of the participants.
Read more on scientific method here: https://brainly.com/question/17216882
Answer:
The company tests three dosages of the mineral supplement on 300 males
and female, between the ages of 25 and 50 over a 6-month period. Each group has 100
Explanation:
Putting your ear to a wall will allows you to hear the noise the other side better than through the air. (True or False) Explain?
Answers
Based on the information given, it should be noted that sound travels through air, and putting your ear to a wall will not allow the person to hear the noise on the other side better. Therefore, it is false.
How does sound travel?It should be noted that when sound is created, the air particles vibrate and collide with each other. The vibrating particles pass the sound through to a person's ear and then vibrate in the eardrum.
Sound travels through air, and putting your ear to a wall will not allow the person to hear the noise on the other side better. In such a case, the noise will be better heard when it travels through the air.
In conclusion, sound needs a medium such as air to travel.
Learn more about sound on:
https://brainly.com/question/9349349
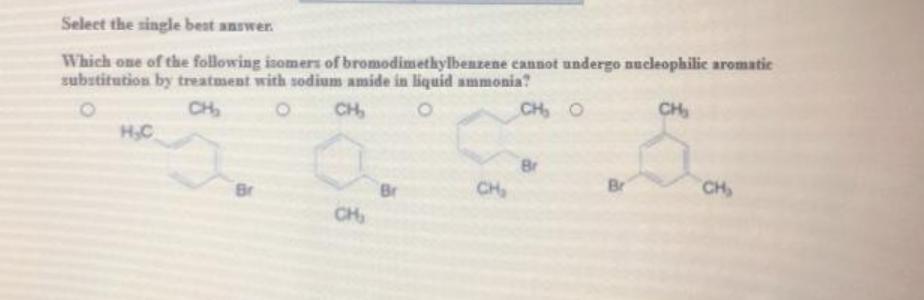
You received no credit for this question in the 16 1 attempt let Check my work 0.75 points Select the single best answer. Which one of the following isomers of bromodimethylbenzene cannot undergo nucleophilic aromatic substitution by treatment with sodium amide in liquid ammonia? CH CH, CH, o сн. H.C pr Br CH References B Br CH CH S Prov o EI Type here to search
Answers
The correct answer is B.Bromodimethylbenzene B cannot undergo nucleophilic aromatic substitution
By treatment with sodium amide in liquid ammonia because the two bromine atoms attached to the benzene ring are meta-directing, which would prevent the reaction from occurring.Bromodimethylbenzene is an organic compound made up of a benzene ring with two bromine atoms and two methyl groups attached. It is a colorless liquid at room temperature and has a slightly sweet odor. It is used as an intermediate in the production of a variety of organic compounds, such as dyes and pharmaceuticals. It can also be used as a solvent for various compounds. Bromodimethylbenzene is a toxic substance and should be handled with caution.
learn more about methyl groups Refer:brainly.com/question/13408577
#SPJ4
The standard entropy of a substance refers to its entropy at:__________.
a. absolute zero and 1 bar
b. 0°C and 1 bar
c. 25 °C and 1 bar
d. 25 °C and 0 bar
Answers
Answer:
b. 0°C and 1 bar
Explanation:
Hello,
In this case, the STP conditions are standard temperature and pressure sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data, it means that a specific pressure and temperature is assigned to analyze the properties of a substance. Such conditions are strictly 0°C and 1 bar because a large number of physical, chemical and thermodynamic properties are measured at them, therefore the standard entropy of a substance refers to its entropy at: b. 0°C and 1 bar.
Best regards.
does a location have an effect on the height of tides
Answers
what is a valency according to chemistry
Answers
Answer:
Valency is the measure of the combining power of an element
1- For endothermic reactions AH>0.
Answers
For an endothermic reaction, ΔH is positive (>0).
For an exothermic reaction, ΔH is negative (<0).
When the temperature of a system rises as a result of the evolution of heat, an exothermic process takes place. The heat of reaction is reduced as a result of the discharge of this heat into the environment.When the temperature of an isolated system drops while the surroundings of a non-isolated system warm up, this is known as an endothermic response. The heat of reaction is generally positive in endothermic reactions.Enthalpy (ΔH), the total of all potential and kinetic energies, varies as a result of energy level disparities between exothermic and endothermic reactions. In a reaction, the system itself determines ΔH, not the environment.Learn more about thermodynamics here:
brainly.com/question/13059309
#SPJ9
when coal goes through combustion it releases nitrogen and sulpur into the air in the form of what
Answers
Answer:
Over time, the plant matter transforms from moist, low-carbon peat, to coal, All types of coal also contain sulfur, which, when burned, releases toxic air pollution. Sulfur All this coal comes from mines, which are either underground or run on diesel—a major source of air toxins, nitrogen oxide, and soot.
List the 5 characteristics of a
physical change
Answers
Answer:
A physical change involves a change in physical properties. Examples of physical properties include melting, transition to a gas, change of strength, change of durability, changes to crystal form, textural change, shape, size, color, volume and density ( is this it )
question: Looking at the above visual of "Atmospheres of the Solar System" What are two patterns
you can find? PLEASE HELP DUE TONIGHT!

Answers
Answer:
The first and most obvious pattern I see is that all gas giants have an atmosphere mainly made up of hydrogen
The second thing I notice is that all the rocky planets have a significant amount of Nitrogen in their atmospheres except Mercury.
The third thing I notice is that all planets have one element that makes up 75% or more of its atmosphere except Mercury.
(If this is good, may I have brainliest, please? I'm kinda poor..)
Answer:
It's hard to find patterns in the solar system's atmosphere but I think above found a lot of them.
the molar solubility of Zn(OH)2 is 5.7x 10^-3 mol/L at a certain temperature. Calculate the value of Ksp for Zn(OH)2 at this temperataure
Answers
Answer:
Ksp = 7.4x10⁻⁷
Explanation:
Molar solubility of a substance is defined as the amount of moles of that can be dissolved per liter of solution.
Ksp of Zn(OH)₂ is:
Zn(OH)₂(s) ⇄ Zn²⁺ + 2OH⁻
Ksp = [Zn²⁺] [OH⁻]²
And the molar solubility, X, is:
Zn(OH)₂(s) ⇄ Zn²⁺ + 2OH⁻
⇄ X + 2X
Because X are moles of substance dissolved.
Ksp = [X] [2X]²
Ksp = 4X³
As molar solubility, X, is 5.7x10⁻³mol/L:
Ksp = 4X³
Ksp = 4 (5.7x10⁻³mol/L)³
Ksp = 7.4x10⁻⁷From top to bottom within a group of the periodic table, first ionization energy tends to *
A. increase.
B. decrease.
C. remain approximately the same.
D. change in an irregular way.
Answers
Answer:
B. decrease.
Explanation:
Because size of atom increases so it is easy to remove electron
A serving of a particular fruit dessert contains 15.0 g of sugar. If all the sugar is sucrose, C12H22O11 (molar mass = 342), how many molecules of sugar are present in this serving?
Answers
There are 2.64 × 1022 molecules of sucrose present in this serving of fruit dessert containing 15.0 g of sugar.
To determine the number of molecules of sugar present in the serving, we need to calculate the number of moles of sugar and then convert it to the number of molecules.
Given:
Mass of sugar (sucrose) = 15.0 g
Molar mass of sucrose (C12H22O11) = 342 g/mol
First, calculate the number of moles of sugar using the formula:
Number of moles = Mass of substance / Molar mass
Number of moles of sugar = 15.0 g / 342 g/mol ≈ 0.0439 mol
Next, we use Avogadro's number, which states that there are approximately 6.022 × 10^23 molecules in one mole of a substance. Therefore, to find the number of molecules of sugar:
Number of molecules = Number of moles × Avogadro's number
Number of molecules of sugar = 0.0439 mol × 6.022 × 10^23 molecules/mol ≈ 2.64 × 10^22 molecules
Therefore, there are approximately 2.64 × 10^22 molecules of sugar present in this serving.
for more such question on molecules
https://brainly.com/question/24191825
#SPJ8
Which equation is balanced?
O 2Fe +02 → 2Fe2O3
O 3Fe +302 → 3Fe2O3
O 4Fe +302 → 2Fe2O3
O Fe +0₂ Fe₂O3
Answers
Answer:
C) 4Fe + 3O2 → 2Fe2O3
Explanation:
For this question, first you would go through all the options and make sure that there is an equal amount of elements on each side.
For the first option: 2Fe +02 → 2Fe2O3
You have only 2Fe atoms and 2O atoms on your reactant side and 4Fe and 6O atoms on your product side. This is not balanced.
For option b: 3Fe +302 → 3Fe2O3
You have 3Fe and 6O atoms on your reactant side while on your product side you have 6Fe and 6O atoms. This is not balanced.
For option c: 4Fe +302 → 2Fe2O3
You have 4Fe and 6O atoms on your reactant side and 4Fe and 6O atoms on your product side. This is balanced!
For option d: Fe +0₂ → Fe₂O3
You have 1Fe and 2O atoms on your reactant side and 2Fe and 3O atoms on your product side. This is not balanced.
***Remember to multiply the subscript of an element by their coefficient (number in front of molecule) if there is any.
Use the following information to answer questions 1 and 2.
Kate had a Styrofoam cooler, a plastic cooler, and an insulated bag.
insulated bag
Styrofoam™ cooler plastic cooler
She wanted to know which container would keep drinks coolest for the longest period of
time. She chilled three identical glasses of water to 40°F. Then, she placed one of the glasses
in each container and closed the containers. During the next two hours, she opened each
container every
15 minutes and measured the temperature of the water. The results of her
experiment appear on the graph below.
Results of Kate's Experiment
Temperature (°F)
inio A All
64
62
60
58
56
54
52
50
48 awon as Isla
46
44
42
40
15
30
.............
5.2C; 5.2D; 5.2G; 5.5A (H)
tadion
T
di casas
1. Which of the following could Kate
blo Fabritodistam
conclude from her results?
e
TM
45
60
75
Time Elapsed (minutes)
A All of the carriers had equal
gnis insulation. srl: looo a
Jaioa
B The water became warmer in each
ams of the containers.
C The Styrofoam cooler kept the
15qcq water coolest over two hours.
STA
D The insulated bag is the best
container for storing cool drinks.
Deplo22ib
90
105
omsins....
Styrofoam™ cooler
-
- plastic cooler
1516W insulated bag
boa gniled (A)
aldds 8
120
Insed oil O
bas? a
5.2E; 5.5A (H)
(3) 82.208.8
Answers
Answer: The Styrofoam cooler kept the water coolest over two hours.
Explanation: From the graph, it is clear that the temperature of the water in the Styrofoam cooler remained the lowest throughout the experiment, and hence, it can be concluded that the Styrofoam cooler kept the water coolest over two hours. The temperature of the water in the insulated bag and plastic cooler both increased more rapidly than in the Styrofoam cooler, indicating that they are not as effective at keeping drinks cool. Therefore, the answer is option C.
The following initial rate data apply to the raction
F2(g) + 2Cl2O(g) ---> 2FClO2(g) +Cl2(g)
Expt. [F2] (M) [Cl2O] (M) Intitial rate (M/s)
1 0.05 0.010 5 x 10^-4
2 0.05 0.040 2.0 x 10^-3
3 0.10 0.010 1.0 x 10^-3
Which of the following is the rate law (rate equation) for this reaction?
A. rate= k[F2]^2 [Cl2O]^4
B. rate= k[F2]^2 [Cl2O]
C. rate= k[F2] [Cl2O]
D. rate= k[F2] [Cl2O]^2
E. rate= k[F2]^2 [Cl2O]^2
Answers
Answer:
C. rate = k[F₂] [Cl₂O]
Explanation:
Based on the reaction, rate law can be obtained from the initial concentration of reactants thus:
rate = k[F₂]ᵃ [Cl₂O]ᵇ
Where the exponents a and b can be finded doing a experiment changing initial concentrations and seeing how a variation contribute in rate law.
If you analize experiments 1 and 2, the only change is [Cl₂O] (From 0.010 to 0.040, four times more) that changes its concentration in four times. This change produce rate law change from 5x10⁻⁴ to 2.0x10⁻³, also four times. That means the exponent b of [Cl₂O] is 1.
rate = k[F₂]ᵃ [Cl₂O]ᵇ
rate = k[F₂]ᵃ [Cl₂O]¹
Now, comparing experiments 1 and 3, the [F₂] change from 0.05 to 0.10, (Twice), and initial rate change from 5x10⁻⁴ to 1x10⁻³ (Also, twice). That means a = 1 and rate law is:
rate = k[F₂]¹ [Cl₂O]
rate = k[F₂] [Cl₂O]
Thus, right answer is:
C. rate = k[F₂] [Cl₂O]If 10.0 moles of O₂ are reacted with excess NO in the reaction below, and only 8.0 mol of NO₂ were collected, then what is the percent yield for the reaction?
2 NO (g) + O₂ (g) → 2 NO₂ (g)
Answers
Answer:
Explanation:
General Remarks
The balance numbers are the same. (The balance numbers are 2 and 2)
The balance number in front of the O2 is 1.
So for every mole of O2 needed 2 moles of NO2 should be produced.
If 10.0 moles of oxygen is used, then you should get 20 mols of NO2 should be produced.
Formula
% yield = (what was produced / what should have been produced) *100
Givens
What was produced = 7 moles
What should have been produced = 20 moles
Solution
Substitute the givens into the equation
% yield = 8/20 * 100
% yield = 0.40 * 100
Answer: 40%
ch4+br2 ch3br+hbr which type of reaction does this equation represent
Answers
To solve such this we must know the concept of combination reaction. Therefore, the given reaction CH\(_4\)+Br\(_2\) \(\rightarrow\)CH\(_3\)Br+ HBr is a combination reaction.
What is chemical reaction?Chemical reaction is a process in which two or more than two molecules collide in right orientation and energy to form a new chemical compound. The mass of the overall reaction should be conserved. There are so many types of chemical reaction reaction like combination reaction, double displacement reaction.
CH\(_4\)+Br\(_2\) \(\rightarrow\)CH\(_3\)Br+ HBr
The above reaction is a combination reaction. In combination reaction, more than one reactant combine to form a product. Because new chemicals are created during combination reactions, they are often referred to as synthesis.
Therefore, the given reaction is a combination reaction.
Learn more about the chemical reactions, here:
https://brainly.com/question/3461108
#SPJ1
98.1 mL of 5 M potassium hydroxide is mixed with 39.9 mL of 4.5 M Iron (III) acetate resulting in a precipitate of Iron (III) hydroxide. Calculate the theoretical yield in g of iron (III) hydroxide.
Answers
Given:98.1 mL of 5 M potassium hydroxide is mixed with 39.9 mL of 4.5 M Iron (III) acetate resulting in a precipitate of Iron (III) hydroxide.To calculate the theoretical yield in grams of Iron (III) hydroxide, the first step is to balance the chemical equation for the reaction that takes place between potassium hydroxide and iron (III) acetate. 3KOH + Fe(C2H3O2)3 → Fe(OH)3 + 3KC2H3O2The balanced chemical equation for the reaction that takes place between potassium hydroxide and iron (III) acetate can be represented as follows;3KOH + Fe(C2H3O2)3 → Fe(OH)3 + 3KC2H3O2The molar mass of Fe(OH)3 is calculated as follows;Molar mass of Fe(OH)3 = Atomic mass of Fe + (3 x Atomic mass of O) + (3 x Atomic mass of H) = (55.85 g/mol) + (3 x 16 g/mol) + (3 x 1 g/mol) = 106.85 g/molThus the molar mass of Fe(OH)3 is 106.85 g/mol.To determine the theoretical yield of Iron (III) hydroxide we must first determine the limiting reactant (the reactant that is fully consumed in the reaction) among potassium hydroxide and iron (III) acetate.Limiting ReactantIn order to find out the limiting reactant among potassium hydroxide and iron (III) acetate, we will first find out the number of moles of each using the formula;Moles = Concentration x Volume in Liters (L)Moles of KOH = Concentration of KOH × Volume of KOH = 5 M × (98.1 mL/1000 mL) = 0.4905 moles Moles of Fe(C2H3O2)3 = Concentration of Fe(C2H3O2)3 × Volume of Fe(C2H3O2)3 = 4.5 M × (39.9 mL/1000 mL) = 0.17955 molesBased on the balanced chemical equation, the mole ratio of KOH to Fe(C2H3O2)3 is 3:1. Hence, the limiting reactant is Fe(C2H3O2)3 since it is lesser in moles compared to KOH. This means that all of the 0.17955 moles of Fe(C2H3O2)3 will be consumed in the reaction while 0.4905 - (0.17955 x 3) = 0.05145 moles of KOH will be left over after the reaction is complete.The theoretical yield is then calculated using the limiting reactant. We can calculate the number of moles of Fe(OH)3 produced from 0.17955 moles of Fe(C2H3O2)3 using the balanced chemical equation. The mole ratio of Fe(C2H3O2)3 to Fe(OH)3 is 1:1. Hence;Moles of Fe(OH)3 = Moles of Fe(C2H3O2)3 = 0.17955 moles. The mass of Fe(OH)3 is then calculated using the formula;Mass = Number of moles × Molar massMass of Fe(OH)3 = Number of moles of Fe(OH)3 × Molar mass of Fe(OH)3 = 0.17955 moles × 106.85 g/mol = 19.179 gTherefore, the theoretical yield of Fe(OH)3 is 19.179 g.
The theoretical yield of iron (III) hydroxide is 19.19 grams.
What is the theoretical yield of iron (iii) hydroxide?The balanced chemical equation for the reaction between potassium hydroxide and iron (III) acetate is:
3 KOH + Fe(C₂H₃O₂)₃ → Fe(OH)₃ + 3 KC₂H₃O₂
To calculate the theoretical yield of iron (iii) hydroxide, first, we determine the limiting reagent.
The number of moles of each reactant:
Number of moles (n) = Molarity (M) × Volume (V)
For potassium hydroxide (KOH):
n(KOH) = 5 M × 0.0981 L
number of moles = 0.4905 moles
For iron (III) acetate (Fe(C₂H₃O₂)₃):
number of moles = 4.5 M × 0.0399 L
number of moles = 0.17955 moles
Since the stoichiometric ratio is 1:1, the number of moles of Fe(OH)₃ = 0.17955 moles.
The molar mass of Fe(OH)₃ = 106.88 g/mol
Theoretical yield = Number of moles × Molar mass
Theoretical yield = 0.17955 moles × 106.88 g/mol
Theoretical yield= 19.19 grams
Learn more about theoretical yield at: https://brainly.com/question/25996347
#SPJ1
What is the product of the unbalanced equation below?
Ca(s) + O2(g)
A. CaO2(5)
B. Cao(s)
C. 2Ca(s) + O2(9)
D. Ca20(s)
Answers
B. Cao(s) (Calcium oxide)
The balanced equation would be:
Ca(s) + O2(g) --> Cao(s)
It is the product of Calcium oxide.
What is the product of the reaction between sodium and chlorine?The product of the reaction between sodium and chlorine is sodium chloride (NaCl). When sodium (Na) and chlorine (Cl) react, they form an ionic bond, with the sodium atom losing one electron to the chlorine atom. This forms a positively charged sodium ion (Na+) and a negatively charged chloride ion (Cl-), which are held together by electrostatic attraction to form the compound sodium chloride. It is a white crystalline solid and is commonly known as table salt.
To know more about Sodium visit: https://brainly.com/question/24010534
#SPJ1
What is the energy of a photon?
A. A photon has no energy.
B. The energy of a photon is hxc.
c. The energy of a photon is hx 2.
D. The energy of a photon is h* f.
Answers
I WILL GIVE 30 POINTS TO THOSE WHO ANSWER THIS QUESTION RIGHT NOOOO SCAMS PLEASE

Answers
The general gas equation, commonly referred to as the ideal gas law, represents the state of a fictitious ideal gas through an equation. Here the mass of helium gas required to pressurize 86 L tank to 201 atm is 2561.8 g.
According to the ideal gas law, the sum of the absolute temperature of the gas and the universal gas constant is equal to the product of the pressure and volume of one gram of an ideal gas.
The ideal gas equation is given as:
PV = nRT
n = PV / RT
56°C = 329 K
n = 201 × 86 / 0.08206 × 329 = 640.45 mol
Molar mass of 'He' = 4.00 g / mol
Mass = 640.45 × 4.00 = 2561.8 g
To know more about ideal gas law, visit;
https://brainly.com/question/30458409
#SPJ1
Classify the following dienes and polyenes as isolated, conjugated, cumulated, or some combination of these classifications.
a. cycloocta-1,4-diene
b. cycloocta-1,3-diene
c. cyclodeca-1,2-diene
d. cycloocta-1,3,5,7-tetraene
e. cyclohexa-1,3,5-triene (benzene)
f. penta-1,2,4-triene
Answers
Answer:
cycloocta-1,4-diene- isolated double bond
cycloocta-1,3-diene- conjugated double bond
cyclodeca-1,2-diene- cumulated double bond
cycloocta-1,3,5,7-tetraene- conjugated double bond
cyclohexa-1,3,5-triene (benzene) - conjugated double bond
penta-1,2,4-triene- cumulated and conjugated double bond
Explanation:
A conjugated double bond is a system that contains alternate double and single bonds. It is renowned for its unusual stability.
A cumulated double bond is a double bond system involving about three atoms e.g C=C=C.
An isolated double bond is a system of double bonds separated by more than one single bond.
Calculate the number of grams in: 2.93moles NaOH.
Answers
We have that the molar mass of NaOH is 40 g/mol (you can calculate this using the periodic table), so the conversion from moles to grams would be:
\(2.93\text{ moles NaOH}\cdot\frac{40\text{ g NaOH}}{1\text{ mol NaOH}}=117.2\text{ g NaOH}\approx117\text{ g NaOH.}\)There are 117 g of NaOH in 2.93 moles.
Answer:
40-gram Weight
Explanation:
Can anyone help please.......
Answers
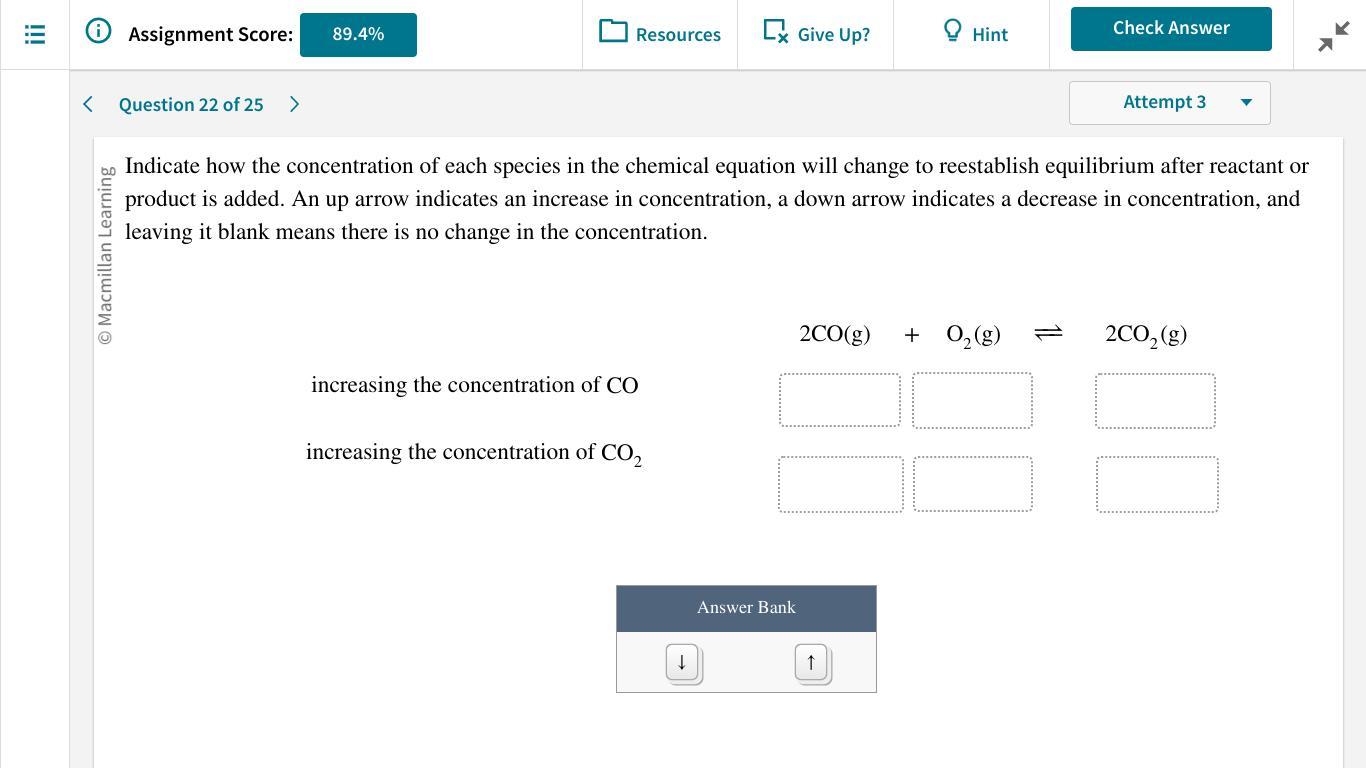
Increasing the concentration of CO decreases the equilibrium concentration of oxygen and increases the concentration of CO₂, increasing the concentration of CO₂ increases the concentration of CO and O₂.
Chemical equilibrium refers to the state of a system in which the concentration of the reactant and the concentration of the products do not change with time, and the system does not display any further change in properties.
It is the state of a reversible reaction where the rate of the forward reaction equals the rate of the reverse reaction. While a reaction is in equilibrium the concentration of the reactants and products are constant.
Learn more about Equilibrium, here:
https://brainly.com/question/30985040
#SPJ1
Answer the questions for 100 Points!

Answers
56) The Aufbau principle has been violated. Option C
57) Hund's rule was violated. Option A
58) This is a valid orbital diagram. Option D
What are the rules for the atom?We know that as we fill the electrons in to the atoms of the elements there bare some rules that we have to observe in the process and all of these rules are the things that make up what we call the Aufbau principle.
In effect one of the rules that we ought to know is the Pauli exclusion principle that states that electrons do not have the same values for all the four quantum numbers.
Learn more about quantum numbers:https://brainly.com/question/16977590
#SPJ1