How exactly are synthetic paint brushes made? Nylon?
Answers
Answer:
Synthetic brushes are typically made from nylon bristles. Other times, they're made from plastic#CARRYONLEARNINGBY : EDLYN MORENO
Related Questions
If you didn't wrap up the condenser with a wet paper towel in the set up for the azeotropic distillation, what might have occurred to cause a lower percent yield?
Answers
Failure to wrap the condenser with a wet paper towel during azeotropic distillation can lead to a lower percent yield due to increased temperature, rapid boiling, formation of bubbles, and loss of solvent and product.
If the condenser was not wrapped up with a wet paper towel during azeotropic distillation, the temperature of the system could increase significantly. This increase in temperature can cause the solvent to boil too rapidly, which can lead to the formation of bubbles in the distillation flask.
These bubbles can trap some of the desired product in the flask, reducing the percent yield. Additionally, if the temperature of the system becomes too high, it can cause the solvent to evaporate too quickly, leading to loss of the solvent and product. This can also reduce the yield of the desired product.
For more such questions on condenser, click on:
https://brainly.com/question/29457387
#SPJ11
A double bond
A. two sigma bonds
B. two pi bonds
C. one sigma and one pi bond
contains

Answers
A double bond consists of one sigma bond and one pi bond.
Therefore Option C is the correct answer.
What is a Double Bond ?A bond in which 4 electrons are shared between a [pair of atoms is called a double bond , higher amount of energy is required to break a double bond.
A double bond consists of one sigma bond and one pi bond.
Therefore Option C is the correct answer.
To know more about Double Bond
https://brainly.com/question/16626126
#SPJ1
How many formula units in 7.25 moles of Al2O3.
Answers
Answer:
If you're asking for the mass (grams)
then 739.21 g
Explanation:
No. of mols = mass/molar mass
Mass= Molar mass x No. of mols
Mass= 101.96 g/mol x 7.25 mols
Mass= 739.21 g
5. An unknown metal of mass 0.75 kg is heated to a temperature of 89.4 degrees Celsius and then
submerged into 1.25 kg of water with an initial temperature of 20.0 degrees Celsius. What is
the specific heat capacity of the metal if the final temperature is 24.4° C? What type of metal
is the unknown sample?
Answers
The specific heat capacity of the unknown metal is 344 J/kg⁰C. And the type of metal that is the unknown sample is silver.
To answer this question, use the following formula:
myCy ΔT= mₓ. Cₓ.ΔT
Information:
mₓ = Unknown mass of metal
ΔTₓ = temperature of unknown metal
my = Mass of water
Cy = Density of water
ΔTy = temperature of water
Given the values for this question.
mₓ = 0.75 kg
ΔTₓ = (89.4⁰C – 0⁰C) = 89.4⁰C
Tₓ = 89.4⁰C
me = 1.25 kg
Cy = 4200J/kg⁰C
ΔTy = (24.4°C - 20.0°C) = 4.4°C
Then the specific heat capacity of the unknown metal is:
Cy ΔTy = Mₓ. Cₓ.ΔTx
Cₓ = Cy ΔTy/ Mₓ.ΔTx
Cₓ =1.25 x 4200 x 4.4/0.75 x 89.4
Cₓ = 23100/67.05
Cₓ = 344 J/kg⁰C
The specific heat of an unknown metal is silver.
Learn more about the specific heat capacity at https://brainly.com/question/28825214.
#SPJ1
Figure 2 shows what happens to the electrons in the outer shells when a sodium atom reacts with a fluorine atom.
The dots (•) and crosses (x) represent electrons.
Describe, as fully as you can, what happens when sodium reacts with
fluorine to produce sodium fluoride.

Answers
Sodium reacts with fluorine to make a covalent bond between them. A covalent bond is formed when there is a mutual sharing of electrons between the two adjacent atoms.
Here, Sodium is metal and fluorine is non-metal. So, the bond between them may be an ionic bond, but as the Sodium has one valence electron and fluorine is highly electronegative. Sodium wants to lose electrons and fluorine wants to gain electrons to form a chemical bond. Therefore, the sodium donates its valence electron to fluorine, forms a cation, and fluorine accepts the electron and forms an anion and they distribute the difference in the charge between them.
So, we can say that when sodium and fluorine react to form sodium fluoride with a covalent bond.
Learn more about covalent bonds here:
https://brainly.com/question/12732708
#SPJ10
Ahh, help me I don’t know what to pick
Answers
Answer:
for what? is there supposed to be a picture?
Explanation:
??
You are task to make sandwiches for your class of 15 students, you are given a sliced bread with 46 slices, and a pack of slice cheese with 16 slice. How many sandwiches can you make?
Answers
To determine the number of sandwiches that can be made with 46 slices of bread and 16 slices of cheese, we need to find the limiting factor between the two.
Since each sandwich requires one slice of bread and one slice of cheese, we can calculate the maximum number of sandwiches by dividing the number of bread slices by 1 and the number of cheese slices by 1. The smaller result would be the limiting factor.
So, the limiting factor is the cheese with 16 slices. Therefore, we can make only 16 sandwiches using all the cheese. Now, let's calculate the number of sandwiches we can make using the 46 slices of bread: If one sandwich requires one slice of bread, then the 46 slices can make 46 sandwiches. Therefore, we can make only 16 sandwiches using the 16 slices of cheese and 46 sandwiches using the 46 slices of bread. In conclusion, we can make 16 sandwiches. We will have 30 slices of bread left unused after making the sandwiches.
To know more about limiting visit:
https://brainly.com/question/12211820
#SPJ11
Calculate the amount (in moles) of gas which occupies 250cm³ at STP
Answers
Answer:
0.01
Explanation:
volume of 22.4 L (22,400 ml) at s.t.p. 250 ml out of 22,400 ml is 0.01 rounded, therefore I assume that is the answer. About 0.01 mole occupies 250 cm3 at s.t.p.
Hydrogen (H), oxygen (O), and Nitrogen (N) are all examples of which of the following?
Question 2 options:
all of these are correct
elements
compounds
molecules
Answers
Answer:
All are correct
Explanation:
This might be a little deceptive. The question shows H, O and N which would designate ELEMENTS.
However, all of these can also be considered compounds and molecules as well. BUT, they are H₂, O₂ and N₂ when they are molecules and compounds.
So it depends on the whether the question is being literal or not.
Translucent and unstainedIndicated by the prescence of a halo between cell and stained background
Answers
The translucent and unstained cells can be identified by the presence of a halo or clear zone between the cell and the stained background. This halo is caused by the difference in refractive index between the cell and the surrounding medium, which causes light to scatter and create a translucent appearance.
This phenomenon is commonly observed in histology and microscopy, where it is used to distinguish between stained and unstained cells. In addition to being a useful diagnostic tool, the presence of a halo can also provide insights into the structure and composition of cells. For example, cells with thicker cell walls or membranes may appear opaquer and have a smaller halo, while cells with thinner walls or membranes may appear more translucent and have a larger halo. Similarly, changes in the size or shape of the halo can indicate changes in the cell's morphology or physiology. Overall, the presence of a halo is an important aspect of cellular analysis that can provide valuable information about the properties of cells and their interactions with the surrounding environment. By understanding the underlying mechanisms that cause this phenomenon, researchers and clinicians can use it to gain insights into a wide range of biological processes and diseases.
learn more about translucent here.
https://brainly.com/question/18124680
#SPJ11
An effective treatment for some cancerous tumors involves irradiation with "fast" neutrons. The neutrons from one treatment source have an average velocity of 3.5×107 m/s. If the velocities of individual neutrons are known to within 2.0% of this value, what is the uncertainty in the position of one of them?
Answers
Answer:
4.54 * 10^-14 m
Explanation:
From Heisenberg's uncertainty principle, we can write;
Δp.Δx ≥ h/4π
Given that;
Δp =Δmv
So;
Δmv.Δx ≥h/4π
Δmv =2/100(3.5×10^7 m/s * 1.66 * 10^-27)
Δmv =1.162 * 10^-21 Kgms-1
Δx ≥6.63 * 10^-34 /4 * 3.142 * 1.162 * 10^-21
Δx ≥4.54 * 10^-14 m
What is the strongest force that exists between molecules of nitrogen
monoxide (NO)?
Answers
Answer:
Hydrogen Bonding (H-Bonding)
Explain the relationships among eons, eras, epochs, and periods of the geologic time scale
Answers
The relationship between them is that they are all times in the geologic scale but have different spans.
The Eons is regarded as the largest unit of time in this scale. The Eon is
divided into smaller part known as Era. The Era is a unit of time which is
shorter than Eon.
Eras is further divided into periods. The period is a unit of time which is
smaller than the era. The Periods are then subdivided into even smaller
time spans known as epochs. This simply means in the periods of geologic
time scale, the eons is the largest time scale and the epoch is the lowest
time scale. The order is shown below
Eons > Era > Period > Epoch
4 NH3 + 7 O2 → 4 NO2 + 6 H2O What is the mole ratio between oxygen and nitrogen dioxide? 7 moles to 6 moles 4 moles to 6 moles 7 moles to 4 moles 4 moles to 4 moles
Answers
Answer:
7:4
Explanation:
O2 : NO2
7 : 4
hope this helps :)
If you triple the force on an object , the acceleration will
a
reduced by 1/3
b
double
c
tripled
d
halve
Answers
Answer:
C triple
Explanation:
please crown me
What are some ways carbon dioxide enters our atmosphere and subsequently the air we breathe
Answers
Answer:
when organisms decompose, or burning fossil fuels and pollution and such
Explanation:
Fruit fly cells have 8 chromosomes. After mitosis, you would expect a resulting fruit fly daughter cell to have how many chromosomes? Explain your answer.
Answers
After mitosis, it is expected that the daughter cells of a fruit fly with 8 chromosomes should have 8 chromosomes.
MITOSIS:
Mitosis is the kind of cell division in which a single cell produces two daughter cells that are genetically identical to the parent cell. Mitosis results in daughter cells that possess the same number of chromosomes as the parent cell, hence, it is called duplication division. This means that mitotic division maintains the chromosome number of parents in the daughter cells. For example, a fruit fly with 8 chromosomes will produce daughter cells with 8 chromosomes each. Therefore, after mitosis, it is expected that the daughter cells of a fruit fly with 8 chromosomes should have 8 chromosomes.Learn more at: https://brainly.com/question/3327479?referrer=searchResults
enough of a monoprotic acid is dissolved in water to produce a 1.48 m solution. the ph of the resulting solution is 2.80 . calculate the ka for the acid.
Answers
The Ka value for the monoprotic acid on dissociation is found to be 16.44.
The dissociation of monoprotic acid HA is as,
HA = H⁺ + A⁻
So, the Ka value is defined as,
Ka = [H⁺][A⁻]/[HA]
The pH of a compound is given as,
log([H⁺]) = pH
The given pH is 2.8, so,
log([H⁺]) = 2.8
[H⁺] = 16.44 M.
Now, as we can see from the equation,
[H⁺] = [A⁻] = [HA]
So, putting the values for Ka,
Ka = [H⁺][A⁻]/[HA]
Ka = 16.44×16.44/16.44
Ka= 16.44
So, the value of Ka for this acid is 16.44.
To know more about pH value of the solution, visit,
https://brainly.com/question/172153
#SPJ4
Michael Faraday is known for his discovery of _______ .
Answers
Michael Faraday discovered electromagnetic induction. This discovery changed the field of electrical power generation and transmission, shaping modern electrical technologies.
Molarity of Kool Aid solutions can be calculated by comparing the concentrations of Kool Aid powder and sugar added to a given volume of water. The molar mass of Kool Aid will be the same as that of sugar for our purpose. The molecular formula for sugar is C12H22O11- Your objective for this lab will be to calculate the molarity of Kool Aid desired based on package directions. You will then be provided two concentrated Kool Aid solutions. You will use dilution calculations to determine the amount of water and concentrated solution you will need in order to prepare 65 mL of the desired molarity.
Calculate the molarity of Kool Aid desired based on the following information from the package directions.
1 package Kool Aid powder = 4. 25 grams 1 cup sugar = 192. 00 grams
2. 00 quarts of water (1. 06 quarts = 1 liter)
Answers
The amount of concentrated solution needed is (0.286 M)(65 mL) / C M, and the amount of water needed is 65 mL minus the volume of the concentrated solution.
To calculate the molarity of Kool Aid desired, we need to determine the number of moles of Kool Aid powder and sugar in the package. Since the molecular formula for sugar is C12H22O11, we can calculate its molar mass as follows:
Molar mass of C12H22O11 = (12 * 12.01) + (22 * 1.01) + (11 * 16.00)
= 144.12 + 22.22 + 176.00
= 342.34 g/mol
Given that the package contains 4.25 grams of Kool Aid powder, we can calculate the number of moles of Kool Aid powder using its molar mass:
Number of moles of Kool Aid powder = Mass / Molar mass
= 4.25 g / 342.34 g/mol
≈ 0.0124 mol
Similarly, for the sugar, which has a molar mass of 342.34 g/mol, we can calculate the number of moles of sugar using its mass:
Number of moles of sugar = Mass / Molar mass
= 192.00 g / 342.34 g/mol
≈ 0.5612 mol
Now, to calculate the molarity of the desired Kool Aid solution, we need to determine the volume of water. Given that 1.06 quarts is equal to 1 liter, and we have 2.00 quarts of water, we can convert it to liters as follows:
Volume of water = 2.00 quarts * (1.06 liters / 1 quart)
= 2.12 liters
To find the molarity, we use the formula:
Molarity (M) = Number of moles / Volume (in liters)
Molarity of Kool Aid desired = (0.0124 mol + 0.5612 mol) / 2.12 L
≈ 0.286 M
To prepare 65 mL of the desired molarity, we can use dilution calculations. We need to determine the volume of concentrated solution and the volume of water needed.
Let's assume the concentration of the concentrated Kool Aid solution is C M. Using the dilution formula:
(C1)(V1) = (C2)(V2)where C1 is the initial concentration, V1 is the initial volume, C2 is the final concentration, and V2 is the final volume.
Given that C1 = C M and V1 = V mL, and we want to prepare a final volume of 65 mL (V2 = 65 mL) with a final concentration of 0.286 M (C2 = 0.286 M), we can rearrange the formula to solve for the volume of the concentrated solution:
(C M)(V mL) = (0.286 M)(65 mL)
V mL = (0.286 M)(65 mL) / C M
So, the amount of concentrated solution needed is (0.286 M)(65 mL) / C M, and the amount of water needed is 65 mL minus the volume of the concentrated solution.
For more such questions on concentrated visit:
https://brainly.com/question/28564792
#SPJ8
would pentane, , be more soluble in ethanol, , or in benzene, ?
Answers
Pentane would be more soluble in benzene than in ethanol.
Pentane is an organic compound that consists of five carbon atoms and twelve hydrogen atoms. It is an alkane that is highly non-polar, making it hydrophobic in nature. It does not dissolve well in water, which is a polar solvent. It has a relatively low boiling point and is highly volatile, making it an excellent solvent for organic compounds. Ethanol is a polar solvent that has the ability to dissolve both polar and non-polar substances.
Benzene is also an organic compound that consists of six carbon atoms and six hydrogen atoms. It is a highly non-polar substance, which is why it does not dissolve well in water. Benzene is an aromatic hydrocarbon, which means it contains a conjugated ring of atoms. It is an excellent solvent for non-polar substances, including pentane.Therefore, pentane would be more soluble in benzene than in ethanol.
Learn more about hydrogen atoms at:
https://brainly.com/question/10806929
#SPJ11
What is the element whose electronic configuration is: \(1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 4 s^2 3 d^{10} 4 p^6 5 s^2 4 d^{10} 5 p^1\) ?
Answers
Answer:
Indium (49)
Explanation:
You can also add all the small numbers up to find the atomic mass of the element, in this case, all the small numbers add up to 49, so if you look for 49 on a periodic table you will find that it is Indium.
Plz help it’s for chemistry!!

Answers
Answer:
Oxygen is negative charge
Explanation:
And The 2 Hydrogens are positive charges
the amount of matter in an object is called
Answers
Answer: Matter
Explanation:
Matter is anything that has volume and/or mass.
Consider the reaction in chemical equilibrium. COCl2(g) Double headed arrow. CO(g) Cl2(g) Which is the correct equation for K? Upper K = StartFraction left-bracket Upper C Upper O Upper C l Subscript 2 Baseline right-bracket Superscript 2 Over left-bracket Upper C Upper O right-bracket left-bracket Upper C l Subscript 2 Baseline right-bracket EndFraction Upper K = StartFraction left-bracket Upper C Upper O Upper C l Subscript 2 Baseline right-bracket Over left-bracket Upper C Upper O right-bracket left-bracket Upper C l Subscript 2 Baseline right-bracket EndFraction Upper K = StartFraction left-bracket Upper C Upper O right-bracket left-bracket Upper C l Subscript 2 Baseline right-bracket Over left-bracket Upper C Upper O Upper C l Subscript 2 Baseline right-bracket EndFraction Upper K = StartFraction left-bracket Upper C Upper O right-bracket left-bracket Upper C l Subscript 2 Baseline right-bracket Over left-bracket Upper C Upper O Upper C l Subscript 2 Baseline right-bracket Superscript 2 EndFraction.
Answers
The correct equation for equilibrium constant is K = [CO][Cl₂] / [COCl₂].
How we calculate the value of K?Here K is the equilibrium constant and value of K is define as the ratio of the product of the concentration of the formed products to the product of the concentration of the reactants.
Given chemical reaction is:
COCl₂(g) ⇄ CO(g) + Cl₂(g)
In the equilibrium state, equilibrium constant will be written as:
K = [CO][Cl₂] / [COCl₂]
Hence, the correct equation for the value of the equilibrium constant is K = [CO][Cl₂] / [COCl₂].
To know more about equilibrium constant, visit the below link:
https://brainly.com/question/1619133
Answer:
Explanation:
b
Consider the following reaction: X
e
(
g
)
+
2
F
2
(
g
)
→
X
e
F
4
(
g
)
. A reaction mixture initially contains 2.24 atm X
e
and 4.27 atm F
2
. lf the equilibrium pressure of X
e
is 0.34 atm, how do you find the equilibrium constant (
K
p
) for the reaction?
Answers
The equilibrium constant (Kp) for the given reaction is approximately 0.489.
To find the equilibrium constant (Kp) for the given reaction, we need to use the partial pressures of the reactants and products at equilibrium. The equilibrium constant expression for gases involves the partial pressures raised to the power of their stoichiometric coefficients. In this case, the stoichiometric coefficients are 1 for Xe and 2 for F2. Given the initial pressures of Xe and F2, as well as the equilibrium pressure of Xe, we can use these values to calculate the equilibrium constant (Kp) for the reaction.
The equilibrium constant (Kp) is given by the expression:
Kp = (P(XeF4)) / (P(Xe) * P(F2)^2)
We are given the equilibrium pressure of Xe (P(Xe)) as 0.34 atm. The initial pressures of Xe and F2 are 2.24 atm and 4.27 atm, respectively. We need to determine the equilibrium pressure of XeF4 (P(XeF4)).
Since the stoichiometric coefficients for Xe and F2 are 1 and 2, respectively, the equilibrium pressure of XeF4 can be represented as 2 * P(Xe) * P(F2)^2. Plugging in the values, we have:
P(XeF4) = 2 * (0.34 atm) * (4.27 atm)^2
Calculating the expression gives us:
P(XeF4) ≈ 9.767 atm
Now that we have the equilibrium pressures of XeF4, Xe, and F2, we can substitute these values into the equilibrium constant expression:
Kp = (9.767 atm) / (0.34 atm * (4.27 atm)^2)
Simplifying the expression yields:
Kp ≈ 0.489
Therefore, the equilibrium constant (Kp) for the given reaction is approximately 0.489.
To learn more about equilibrium click here:
brainly.com/question/30694482
#SPJ11
which element has the highest monetary value?
A) gold
B) Silver
C) nickel
D) lead
Answers
Answer:
A. gold has the highest monetary value?
Among the given elements, gold has the highest monetary value. So the correct option is A.
What is gold?
Gold (Au) is a chemical element that belongs to Period 6's Group 11 (Ib) and is a thick, glossy golden valuable metal. Gold has historically been extremely valued due to a number of characteristics. It is typically found in nature in a relatively pure form, is appealing in colour and brightness, resilient to the point of virtual indestructibility, and very flexible.
Due to its perceived worth from the beginning, gold has a history that is unmatched by that of any other metal. Gold is one of the densest metals.
It is a good heat and electrical conductor. It is also the softest, most malleable, and ductile of all the elements; a troy ounce (31.1 grammes) of gold may be hammered into gold leaf, which can be crushed into sheets as thin as 187 square feet (approximately 17 square metres).
Therefore the correct option is A.
Read more about gold, here
https://brainly.com/question/4838993
#SPJ2
the theoretical yield is 536.1 g CO2 and the actual yield is 164.1 g CO2 what is the percent yield ?
Answers
Report the following measurement using three sig figs?
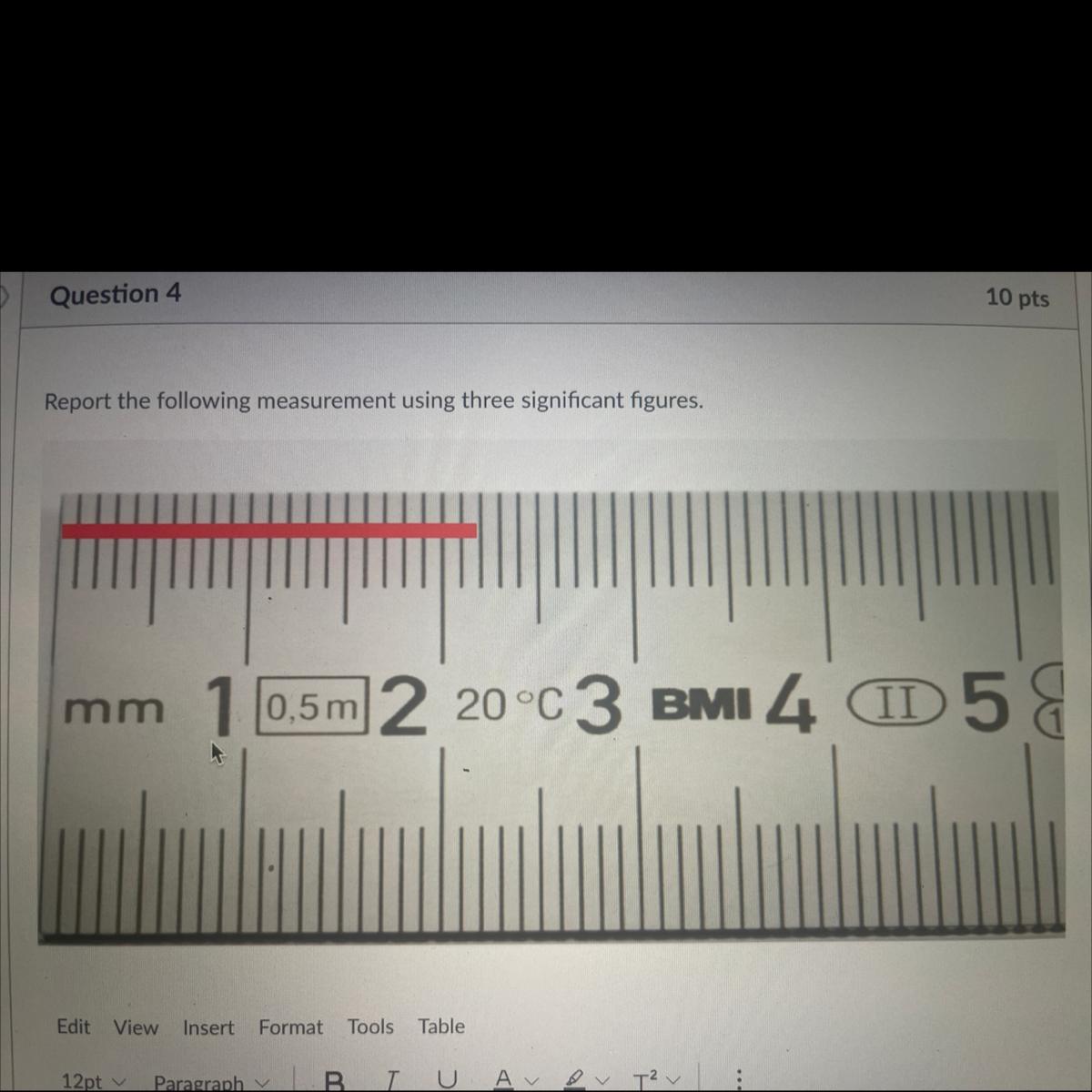
Answers
Answer:
the answer is
2.19
hope this will help you ❤️
The "proton pumps" indicated in the figure are physically associated with ______.a. the electron transport chainb. the Krebs cyclec. the ATP synthased. glycolysis
Answers
The "proton pumps" indicated in the figure are physically associated with the electron transport chain.
What are Proton Pumps?The proton pumps are embedded within the inner mitochondrial membrane and play a crucial role in generating the proton gradient, which drives ATP synthesis through ATP synthase.
The "proton pumps" are part of the electron transport chain (ETC). The ETC is a series of protein complexes located in the inner mitochondrial membrane that transport electrons from electron donors (such as NADH and \(FADH_{2}\)) to electron acceptors (such as oxygen) through a series of redox reactions. As electrons pass through the ETC, protons are pumped from the mitochondrial matrix to the intermembrane space, creating a proton gradient that is used to generate ATP by the ATP synthase enzyme.
To know more about Proton Pumps:
https://brainly.com/question/31147421
#SPJ11