Answers
Answer:
I think I know what you're supposed to do here. You're supposed to fill out the blanks using at least one given answer. For example, on number 15, you should be able to find out the ions and the formula using the names of the substances. On number one, you should be able to figure out the formula and the names using the ions given.
Explanation:
Given above.
Related Questions
if the solubility of a solid substance is 18.2 g/100 g water, which of the following best describes what eventually forms after 10.0 grams of the substance is mixed with 50.0 grams of water?
Answers
When 10.0 grams of a substance with a solubility of 18.2 g/100 g water is mixed with 50.0 grams of water, a saturated solution is formed. This means that the maximum amount of the substance that can dissolve in the given amount of water has been reached, and any additional substance will not dissolve, resulting in a solid residue.
The solubility of a substance indicates the maximum amount of that substance that can dissolve in a given amount of solvent under specific conditions, usually at a particular temperature. In this case, the solubility is given as 18.2 g/100 g water.
When 10.0 grams of the substance is mixed with 50.0 grams of water, the total amount of water is 50.0 grams, which is less than the solubility of 18.2 g/100 g water. As a result, a saturated solution is formed, where the maximum amount of the substance that can dissolve in the given amount of water has been reached.
Any additional substance that is added beyond the solubility limit will not dissolve and will remain as a solid residue at the bottom of the container. Therefore, after mixing 10.0 grams of the substance with 50.0 grams of water, a saturated solution is formed, and the remaining substance will form a solid residue.
To learn more about solubility visit: brainly.com/question/28170449
#SPJ11
Are the following chemical equations balanced or
Balanced / Onbalanced
.
CH4 + 4Cl2 → CCL + 2HCL
Balanced / Unbalanced
N2 + 3H2
2NH3
Balanced / Unbalanced
C3H2 + 302 + 3CO2 + 4H2O
+
Answers
Answer:
the first is unbalanced
because the number of hydrogen are not equal on both sides
the second one is balanced
because the number of nitrogen and hydrogen atoms are equal on both sides
and the third one seems to be incomplete
Chemical Energy
Chemical energy is the energy that substances possess because of the way their _________ are ___________
together. The ___________ of chemical energy is different for different substances and determines whether
energy is absorbed or released during a chemical reaction:
❖ If reactants have more chemical energy than products, energy is absorbed released.
❖ If reactants have less chemical energy than products, energy is absorbed released.
Activation Energy
All chemical reactions need a certain amount of energy to begin. This energy is called ____________
___________.
• The activation energy is often _____________ energy, but it can also be any other form of energy.
• The amount of activation energy needed is always the same for a particular reaction, unless a catalyst
is used.
o A catalyst is a special substance that ___________ activation energy.
Why is activation energy needed?
Reaction Energy Diagrams
A reaction energy diagram shows the process of a chemical reaction in terms of energy. The images show
these energy values:
➢ _______________________________________________________
➢ _______________________________________________________
➢ __________________________
fill in the blanks
Answers
Chemical energy is the energy that atoms have due of how they are bound together. varied substances have varied amounts of chemical energy, which impacts whether energy is absorbed or released during a chemical reaction.
The energy necessary for a chemical reaction to begin is known as activation energy. It is often thermal energy, although it can be any type of energy. Unless a catalyst is utilised, the quantity of activation energy required for a certain reaction is always the same.
A catalyst is a unique material that minimises the amount of energy required for activation. Reaction energy diagrams depict the energy flow of a chemical reaction. The illustrations depict the following energy values: • The energy of the reactants • The energy of the reactants • The activation energy • The energy of the products.
Learn more about energy at:
https://brainly.com/question/1932868
#SPJ1
Use the Lewis model to determine the formula for the compound that forms from each pair of atoms.
Express your answer as a chemical formula.
1) Sr and S
2) Mg and Cl
3) Na and I
Answers
The Lewis model is a method to predict the formation of a chemical bond between atoms. It involves determining the number of valence electrons in each atom and then pairing them up to form a bond.
How do you express the answer as a chemical formula for the given elements? Sr and S:Sr has 2 valence electrons, while S has 6 valence electrons. To form a compound, Sr must lose its two valence electrons, while S must gain two electrons. The resulting compound will have the same number of positive and negative charges, which will cancel out. Therefore, the chemical formula for the compound formed between Sr and S is SrS.
Mg and Cl:Mg has 2 valence electrons, while Cl has 7 valence electrons. To form a compound, Mg must lose its two valence electrons, while Cl must gain one electron. However, Cl cannot gain two electrons to form a stable compound. Therefore, Mg must lose both of its valence electrons to form a compound with Cl. The resulting compound will have one positive charge (from Mg) and one negative charge (from Cl), which will cancel out. Therefore, the chemical formula for the compound formed between Mg and Cl is MgCl2.
Na and I:Na has 1 valence electron, while I has 7 valence electrons. To form a compound, Na must lose its valence electron, while I must gain one electron. The resulting compound will have one positive charge (from Na) and one negative charge (from I), which will cancel out. Therefore, the chemical formula for the compound formed between Na and I is NaI.
Learn more about Lewis structure here:
https://brainly.com/question/20300458
#SPJ1
Tiles
fluorine
Pairs
aluminum
loses one electron
loses two electrons
gains three electrons
loses three electrons
gains one electron
gains two electrons
phosphorus
sodium
calcium
sulfur

Answers
Here is the pairing of elements with their respective electron behaviors:
Fluorine: Gains one electronAluminum: Loses three electronsPhosphorus: Gains three electronsSodium: Loses one electronCalcium: Loses two electronsSulfur: Gains two electronsWhat are electron loss and electron gain?Electron loss and electron gain refer to the transfer of electrons between atoms during chemical reactions, specifically in the formation of chemical bonds.
Electron loss and electron gain are fundamental processes in chemical reactions, as they allow atoms to achieve a more stable electron configuration by attaining a full valence shell, similar to the noble gases. This transfer of electrons leads to the formation of ionic bonds between positively and negatively charged ions or can contribute to the formation of covalent bonds by sharing electrons between atoms.
Learn more about electrons at: https://brainly.com/question/31620742
#SPJ1
Determine the unknown mineral using the information given below. Be sure to use significant figure rules when doing calculations. Mass is shown on the triple beam. Scale Test Mineral Mineral H. Density, g/cc 10 diamond tellurium 2 2.07 9 corundum galena 2.5 7.58 8 topaz anglesite 2.5 - 3 6.4 7 quartz chalcocite 2.5 - 3 5.6 6 feldspar copper 2.5 - 3 9.0 5 apatite gold 2.5 - 3 19.3 4 fluorite silver 2.5 - 3 10.5 3 calcite arsenic 3.5 5.7 2 gypsum barite 3 - 3.5 4.4 1 talc dolomite 3.5 - 4 2.9 platinum 4.5 21.5 willemite 5.5 4.0 magnetite 6 5.18 pyrite 6 - 6.5 5.02 pyrolusite 6 - 6.5 5.0 cassiterite 6.5 6.9 diamond 10 3.52 Volume of water displaced = 0.051 L
Mass: 981.0, 908, 908.1, 981 g
Density: 192.35, 19,235, 19, 19.2 g/cc
Hardness 4, 2.5, 1, 3, 5.5 - 5, 3, 2, 4.5, 1.5
Determine the unknown mineral.
Platinum, Gold
Answers
The unknown mineral is gold because of the similar properties of gold to that unknown mineral.
What is unknown mineral?The unknown mineral is Gold because it has similar properties to the gold. Gold has density of 19.3 grams per cubic centimeter which is same to this unknown mineral. The hardness and mass of the mineral are also similar to the Gold.
So we can conclude that the unknown mineral is gold because of the similar properties of gold to that unknown mineral.
Learn more about mineral here: https://brainly.com/question/15844293
#SPJ1
What concentration of H+ ions does a substance with a pH of 3 have
Answers
Answer: 1 x 10-3 mol/L
Explanation:
Question
What concentration of H+ ions does a substance with a pH of 3 have
the pH is the negative log of the H ion concentration in moles/liter
so pH3 has a hydrogen ion concentration of 1 x 10-3 mol/L
cho lượng dư Al tác dụng với dung dịch H2SO4 đặc nóng. Đâuuf tiên thấy giải phóng ra khí màu A mùi sốc, khí A làm mất màu dung dịch nước brom. Tiếp theo tạo thành kết tủa màu vàng. Rồi thoát ra khí không màu B mùi trứng thối, khí B cũng làm mất màu dung dịch nước brom, tạo kết tủa khi dẫn vào dung dịch Cu(NO3)2. Viết các phương trình phản ứng
Answers
Hope it helps you!
-miraculousfanx-
how much heat (in cal) is required to boil 142 g of ammonia, nh3? the heat vaporization of ammonia is 327cal/g
Answers
It would require 46434 calories of heat to boil 142 g of ammonia.
When a liquid changes form into a gas, the process is called vaporization. You can watch vaporization when you boil a pot of water. Vaporization happens in two ways: evaporation and boiling. Evaporation occurs when sunlight shines on water until it changes to vapor and rises into the air
To calculate the amount of heat required to boil 142 g of ammonia, NH3, we can use the formula:
Heat = mass x heat of vaporization
where the mass is 142 g and the heat of vaporization is 327 cal/g.
Therefore, the heat required to boil 142 g of ammonia is:
Heat = 142 g x 327 cal/g
Heat = 46434 cal
For such more questions on Vaporization
https://brainly.com/question/26306578
#SPJ4
P +
Cl2 →
PC13
Balance the chemical equation
Answers
Answer:
2P + 3Cl2 => 2PCl3
Explanation:
Balance two of the three equations below by adding the
coefficient next to the highlighted element(s). You get to
pick the two equations you want to balance!

Answers
The balanced equation is as follows:
Cr₂O₃ + 3Mg → 2CrO₃ + 3MgO
2H₂ + O₂ → 2H₂O
2NaBr + Cl₂ → 2NaCl + Br₂
What is a balanced chemical equation?A balanced chemical equation is a representation of a chemical reaction using the chemical formulas of the reactants and products. The equation shows the relative amounts of each reactant and product, and it satisfies the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction, only rearranged.
A balanced chemical equation has the same number of atoms of each element on both sides of the equation, meaning that the total mass of the reactants is equal to the total mass of the products. The balancing is achieved by adjusting the coefficients in front of the chemical formulas to ensure that the number of atoms of each element is equal on both sides of the equation.
To know more about reactants, visit:
https://brainly.com/question/17096236
#SPJ1
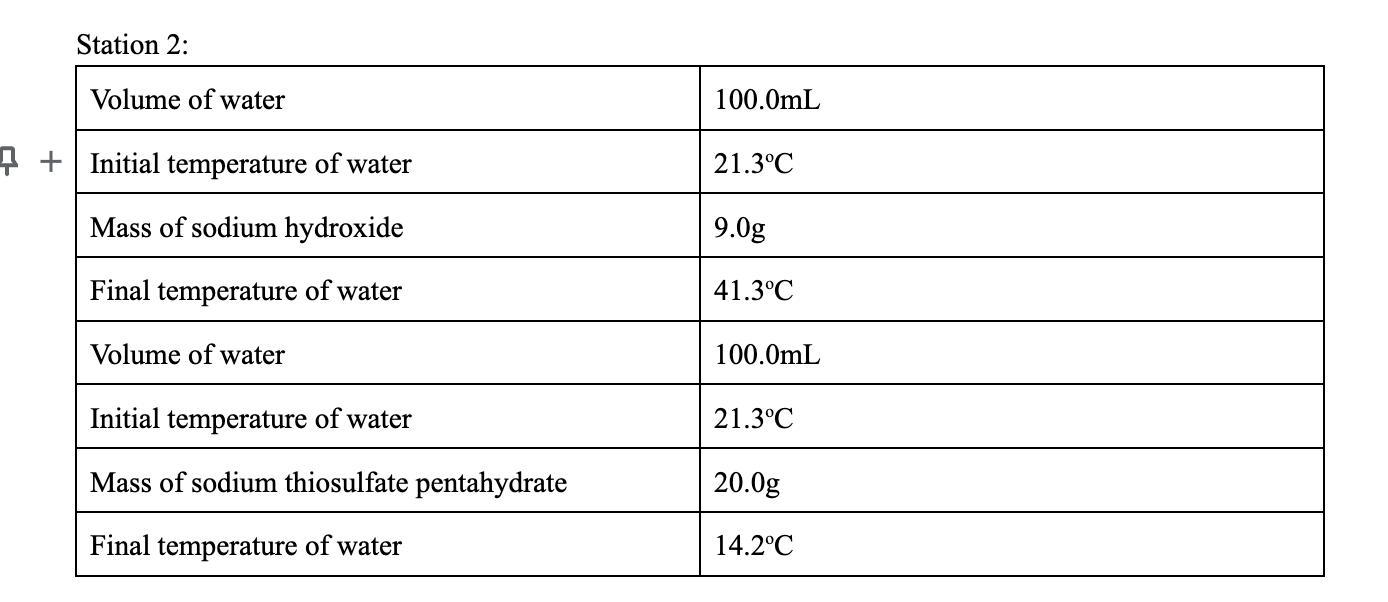
Calculate the heat released or absorbed. Using Q = m * Cp * TCp = 4.186 J/gK
Answers
Explanation:
a) Sodium Hydroxide:
Volume of water = 100.0 mL
Initial temperature of water = 21.3 °C
Mass of NaOH = 9.0 g
Final temperature of water = 41.3 °C
So we had a 100.0 mL sample of water at 21.3 °C and after we added 9.0 g of NaOH the temperature increased to 41.3 °C. Let's find the heat that the water absorbed using the formula:
Q = m * Cp * ΔT
Where m is the mass, Cp is the specific heat of water (Cp = 4.186 J/g°C) and ΔT is the temperature change.
First we had 100.0 mL of water or 100.0 g (if we consider that the density of water is 1.0 g/mL) and we added 9.0 g of NaOH. Let's find the total mass.
m = total mass = mass of water + mass of NaOH
m = 100.0 mL * 1.0 g/mL + 9.0 g
m = 109.0 g
We can also find the change in temperature.
ΔT = Tfinal - Tinitial = 41.3 °C - 21.3 °C
ΔT = 20.0 °C
The dissolution of the NaOH is an exothermic process. This reaction will release heat that will be absorbed by its surroundings and the temperature of the water will increase.
Finally we can calculate the heat that was absorbed.
Qw = m * Cp * ΔT
Qw = 109 g * 4.186 J/(g°C) * 20.0 °C
Qw = 9125 J
So the water absorbed 9125 J and the reaction released -9125 J.
b) Sodium thiosulfate pentahydrate:
Volume of water = 100.0 mL
Initial temperature of water = 21.3 °C
Mass of sodium thiosulfate pentahydrate = 20.0 g
Final temperature of water = 14.2 °C
The dissolution of the sodium thiosulfate pentahydrate is an endothermic process in this case. The dissolution will absorb energy from its surroundings and the temperature of the water will decrease.
m = total mass = mass of water + mass of thiosulfate
m = 100.0 mL * 1.0 g/mL + 20.0 g
m = 120.0 g
ΔT = Tfinal - Tinitial = 14.2 °C - 21.3 °C
ΔT = -7.1 °C
Qw = m * Cp * ΔT
Qw = 120 g * 4.186 J/(g°C) * (-7.1 °C)
Qw = -3566 J
So the water released -3566 J and the dissolution process absorbed 3566 J.
Answer:
a) The water absorbed 9125 J and the dissolution process released -9125 J.
b) The water released -3566 J and the dissolution process absorbed 3566 J.
Can someone help with this pls!!

Answers
Answer:
2
Explanation:
good vibes
Convert 7g/dm^3 of H2O to mol/dm^3
Answers
Conversion of 7g/dm³ of H₂O to 0.21 mol/dm³ is
Conversion is the act or process of changing something into a different state or form
Here given data is
7g/dm³ of H₂O we have to convert it into mol/dm³ = ?
Then divide by the relative formula mass
We get H₂O then H = 1 here 2 hydrogen = 1×2 = 2 and 1 oxygen i.e 1×16 = 16 =
2×16 = 32
So, 7g/dm³/32
= 0.21 mol/dm³
7g/dm³ of H₂O to 0.21 mol/dm³
Know more about convert
https://brainly.com/question/16982880
#SPJ1
cis,cis-hexa-2,4-diene draw the molecule on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars.
Answers
Hexa-2,4-diene-1,6-diol,cis,cis- Muconic alcohol is the name of this Molecule.
What structure does 2-nitrophenol have?The chemical compound 2,4-Dinitrophenol (often known as 2,4-DNP or simply DNP) has the formula HOC6H3(NO2)2. It smells pleasant and musty and is a yellow, crystalline substance. It is soluble in the majority of organic solvents as well as aqueous alkaline solutions, sublimates, and is volatile with steam.
What is henna, 2 amino-4,6 dinitrophenol?Picric acid is a base that can be made into picramic acid, also known as 2-amino-4,6-dinitrophenol, by neutralizing it with an alcoholic solution of ammonium hydroxide. The resulting solution is then treated with hydrogen sulfide, which causes it to turn red and produce sulfur and red crystals.
To know more about Molecule visit:-
https://brainly.com/question/19556990
#SPJ4
Question 10 of 34 >
A sample of water, H2O, has a mass of 56.00 g. Calculate the number of water molecules in the sample.
number of molecules:
molecules
Answers
Moles of Water
Moles = mass ÷ molar mass
= 56.00 g ÷ 18.015 g/mol
= 3.1085 mol
Molecules of Water
Number of molecules = moles × Avogadro's Number
= 3.1085 mol × (6.022 × 10²³ molecules/mol)
= 1.872 × 10²⁴ molecules
A 56.00 g sample of water contains 1.872 × 10²⁴ molecules.Note: We could do all this calculation in one step but I separated it to make it clearer.
Why is air cooled before nitrogen and oxygen are obtained
Answers
Answer:
The main reason why air is cooled before nitrogen and oxygen are obtained is because these two gases are much more soluble in cold air than in warm air. As a result, if air were not cooled before these gases were separated, they would simply mix back together again.
making the simplistic assumption that the dissolved nacl(s) does not affect the volume of the solvent water, determine the constants m and b in the equation density
Answers
The total volume of the solution is 150 mL.
The equation for density is defined as the mass of a substance divided by its volume. In this case, we are making the assumption that the dissolved NaCl(s) does not affect the volume of the solvent water.
To determine the constants "m" and "b" in the equation for density, we need to consider the relationship between mass and volume. Let's use an example to explain this further:
Let's say we have a solution of water and dissolved NaCl(s). The total volume of the solution is 150 mL.
To find the density, we need to know the mass of the solution. Suppose the mass of the solution is 300 g. We can use the equation:
Density = Mass/Volume
Substituting the known values, we get:
Density = 300 g / 150 mL
Now, let's simplify this equation:
Density = 2 g/mL
From this example, we can see that the constant "m" in the equation for density is 2, and the constant "b" is the unit of measurement, which is g/mL in this case.
In summary, the constants "m" and "b" in the equation for density depend on the specific Band its unit of measurement. In the example provided, the constant "m" is 2, and the constant "b" is g/mL.
Learn more about volume on
https://brainly.com/question/14197390
#SPJ11
Where do most fungi thrive?
Answers
Question :
Where do most fungi thrive ?
Answer :
Fungi are found all around the world and grow in a wide range of habitats, including deserts. Most grow on land (terrestrial) environments, but several species live only in aquatic habitats. Most fungi live in either soil or dead matter, and many are symbionts of plants, animals, or other fungi.
Source :
CK-12 , Edgenuity2020
What change was made to the Mendeleev’s early Periodic Table? (How is it currently organized?
A.
They used atomic mass instead of atomic number to organize the elements.
B.
They included chemical properties such as bonding power.
C.
They included physical properties such as melting point and density.
D.
They used atomic number instead of atomic mass to organize the elements.
Answers
Answer:
D
Explanation:
po promise meheehehehehe
Objectives
At the completion of this lab, the student will be able to:
1. Apply the formulas and to determine the output using for the MC-culloch & Pitts neuron model for various logic functions.
2. Run a perceptron model using MATLAB and determine the outputs using various inputs parameters.
Equipment and Materials:
Computer with MATLAB environment
Form a group of three students and perform the simulation in MATLAB
Lab Activity: Simulation
Design and develop the Artificial Neural network model for the following experiments
Experiment 1: McCulloch and Pitts Network
Experiment 2: Hebbian Network
1. Design and train a neural network system which can perform AND and OR operation.
2. Tune the neural network model and minimize the error by updating the weights and perform the testing.
3. Run the simulation in group and explain the working principles of the algorithm. 4. Interpret the output of the designed neural network system by varying the inputs
Answers
The main objective of the lab is to design and develop an Artificial Neural Network model for two experiments: the McCulloch and Pitts Network and the Hebbian Network. The students will design and train a neural network system capable of performing AND and OR operations.
They will also tune the model to minimize errors by updating the weights and conducting testing. The simulation will be run in groups, where the working principles of the algorithm will be explained. The output of the neural network system will be interpreted by varying the inputs.
The lab aims to provide students with practical experience in working with artificial neural networks. In Experiment 1, the students will focus on the McCulloch and Pitts Network and implement it to perform logic operations like AND and OR. They will train the neural network model and update the weights to minimize errors. Through testing, the effectiveness of the designed model will be evaluated.
In Experiment 2, the students will explore the Hebbian Network and its learning principles. They will gain insights into how the network adjusts its connections based on the input and output patterns. The students will analyze the behavior of the network and its ability to learn and adapt.
The lab emphasizes collaborative work, as students are expected to form groups and run the simulation together. This encourages discussion and explanation of the algorithm's working principles among peers. Additionally, varying the inputs and observing the corresponding outputs will allow the students to understand how the neural network system responds to different scenarios and interpret its functioning.
learn more about Hebbian Network here:
https://brainly.com/question/33291958
#SPJ11
What can be said about the number of electrons atoms of one element can have?
Answers
Answer:
The number of protons in one atom of an element determines the atom's identity, and the number of electrons determines its electrical charge. The atomic number tells you the number of protons in one atom of an element.
How many elements are present in the chemical formula CH,₂,0? +
Answers
The chemical formula CH₂O contains 3 elements: carbon, hydrogen, and oxygen.
How many elements are present in the chemical formula CH,₂,0?The chemical formula CH₂O represents formaldehyde, which is a simple organic compound.
To determine the number of elements in this formula, we count the different types of atoms that are present. In this case, there are three different types of atoms: carbon (C), hydrogen (H), and oxygen (O).
Therefore, the chemical formula CH₂O contains 3 elements: carbon, hydrogen, and oxygen.
Learn more about number of elements here: https://brainly.com/question/25916838
#SPJ1
PLEASE HELP!?Consumers must eat other organisms for energy. Which organisms are consumers in this food chain?
Answers
Answer:
Consumers must consume other organisms to get the food that they need and are known as Heterotrophs as they cannot make their own glucose. These consumers eat producers (plants). Herbivores are considered as first order consumers. These consumers eat consumers and producers (animals and plants).
Which one of the following is a main-group metal element in period number 3 of the periodic table?
a. manganese
b. magnesium
c. gallium
d. sulfur
e. boron
f. none of the above
Answers
Magnesium is a main-group metal element in period number 3 of the periodic table, which is the correct answer.
Period number 3 of the periodic table has eight elements that are arranged in order of increasing atomic number. These elements are sodium, magnesium, aluminum, silicon, phosphorus, sulfur, chlorine, and argon.
The period number signifies the highest energy level that the element's electrons occupy.
The metallic character of the elements reduces, and the non-metallic character increases as you move across a period. Magnesium is a silvery-white metal and the eighth most abundant element in the Earth's crust.
Magnesium is used in alloys, pyrotechnics, and flares, and it is also used to manufacture lightweight and durable products like aircraft parts.
Magnesium is a main-group metal element and is located in Group 2 of the periodic table. It is in the same group as beryllium, calcium, strontium, barium, and radium.
It is a highly reactive metal that readily loses its two outermost electrons to form a stable cation with a 2+ charge. Magnesium ions have a high affinity for water molecules, which makes them essential in biological systems as well.
Magnesium is an essential element for human health, and it is involved in many physiological processes, including muscle contraction and relaxation, nerve transmission, and energy metabolism.
Magnesium deficiency can cause a variety of health problems, including muscle weakness, cramps, and irregular heartbeats.
In conclusion, magnesium is a main-group metal element in period number 3 of the periodic table.
To know more about Magnesium, visit:
https://brainly.com/question/8351050
#SPJ11
express the equilibrium constant for the following reaction. 2 ch3cl(g) cl2 (g) ⇔ 2 ch2cl2 (g) h2 (g)
Answers
The value of K will vary depending on the specific conditions of the reaction.
The equilibrium constant, denoted as K, expresses the ratio of the concentrations (or partial pressures) of the products to the concentrations (or partial pressures) of the reactants at equilibrium. For the given reaction:
2 CH₃Cl(g) + Cl₂(g) ⇌ 2 CH₂Cl₂(g) + H₂(g)
The equilibrium constant expression can be written as:
K = [CH₂Cl₂]²[H₂] / [CH₃Cl]²[Cl₂]
Note that the concentrations of gases are usually expressed in terms of their partial pressures. If the concentrations are given in molarities, you can replace them with the corresponding partial pressures using the ideal gas law.
It's important to note that without specific information on the concentrations (or partial pressures) of the substances at equilibrium, it's not possible to calculate the numerical value of the equilibrium constant K. The value of K will vary depending on the specific conditions of the reaction.
Learn more about equilibrium constant from the link given below.
https://brainly.com/question/28559466
#SPJ4
Au HSO3 nomenclatura
Answers
What are the products?

Answers
Answer:
option 3
Explanation:
3 is the answer u get before you balance the equation
What is the mass number of an ion with 109 electrons, 158 neutrons, and a 1 charge?
Answers
The mass number of an ion with 109 electrons, 158 neutrons, and a 1 charge is 267. In atoms, the mass number is the total number of protons and neutrons in the nucleus.
However, ions are atoms that have gained or lost electrons, resulting in a change in their charge. In this case, the ion has a 1 charge, indicating that it has lost one electron. Since the number of protons remains the same for an element, which is determined by its atomic number, we can deduce that the atom originally had 110 electrons to balance the 110 protons. To find the mass number, we add the number of protons (110) and neutrons (158) since they contribute to the overall mass of the atom. Therefore, the ion's mass number is 267 (110 protons + 158 neutrons).
Learn more about mass number here: brainly.com/question/18803094
#SPJ11
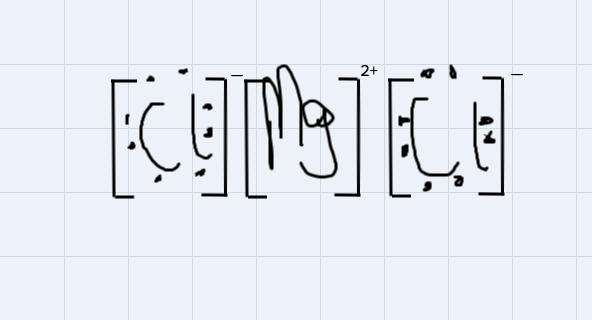
Use Lewis symbols to show how MgCl2 will be formed from Mg and Cl2.
Answers
This is a type of bonding that is formed from the from the attraction of oppositely charged ions in a compound.
For instance, MgCl2 is an ionic compound because the 2 positive ions wipossessed by the magnessium atom will attract each of the negtaive ion possessed by each of the chlorine atom to form the magnessium chloride compound
Using the Lewis symbol to demonstrate the bondng:
From the disgram, the negative ions on chlorine atoms will get attracted to the positive ions on the magnessium ion.