Answers
Answer:
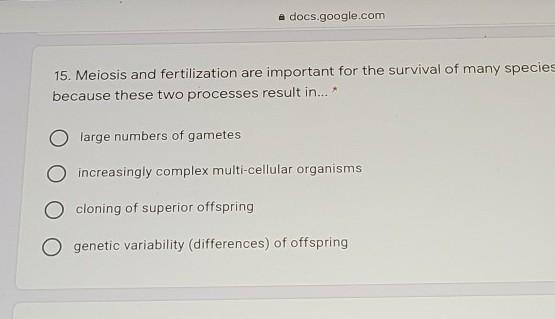
B.
Explanation:
I had this same question today and got it right
Answer:
Genetic variability of offspring
Explanation:
Basically the sorting of genes
Related Questions
If zinc replaces iron, iron replaces lead, and zinc replaces lead in a chemical reactions, the order of activity from most active to least active is
Answers
In the given scenario, the order of activity from most active to least active is zinc, iron and lead.
The activity of an element in a chemical reaction is determined by its ability to participate in the formation of a compound. In this case, zinc is replacing both iron and lead, meaning it is more active than both of those elements and considered to be the most active element. Iron is only replacing lead, making it less active than zinc but more active than lead.
Lead is the only element that is being replaced, making it the least active element among the three. This order of activity is based on the relative ability of these elements to participate in chemical reactions and form compounds.
Read more about the Reactivity of elements:
https://brainly.com/question/19109836
#SPJ4
We apply the same amount of energy to 10.0-g samples of aluminum, iron, and silver which begin at the same temperature. Rank the metals from highest to lowest temperature after the heat is applied
Answers
Answer:
Ag > Fe > Al
Explanation:
The values of the specific heat capacity of the elements are given as;
Aluminum = 0.897 J/g*K
Iron=0.449 J/g*K
Silver=0.235 J/g*K
Specific heat capacity basically refers to how much heat is required to change the temperature of a unit mass of a substance by a degree. Elements with high specific heat capacity would require higher energy compared to elements with lower values,
Aluminium (Al) has the highest specific heat capacity, hence it wold require more energy to cause a change in temperature. Aluminium would be the lowest.
Silver (Ag) has the lowest specific heat capacity hence it would require less energy to cause a change in temperature. Silver wold be the highest.
The middle would be iron (Fe).
The rank is given as;
Ag > Fe > Al
5. What is the molar mass of ethanol (C2H5OH)?
A) 45.06 g/mol
C) 30.03 g/mol
B) 34.06 g/mol
D) 46.07 g/mol
Answers
The molar mass is given by the sum of the atomic masses of the component elements of the substance.
In our case, the substance is ethanol or C₂H₅OH.
\(A_C=12.011 \)
\( A_H=1.0079 \)
\( A_O=15.9994\)
\(\mathcal M_{C_2H_5OH} = 2 \times A_C + 6 \times A_H + A_O = 2 \times 12.011 + 6 \times 1.00079+ 15.999 4= 24.022 + 6.0474 + 15.9994= 46.069≈ \boxed{46.07 \dfrac{g}{mol}}\)
During a chemical reaction, if the concentration of reactants and products doesn't change, the reaction has likely stopped. true false
Answers
According to the research, the correct option is false, the statement related to a chemical reaction is false.
What is a chemical reaction?They are reactions involving two or more substances (reactants), which change significantly in the process, and can consume or release energy.
Every chemical reaction subjects matter to a chemical transformation, altering its structure and molecular composition.
Therefore, we can conclude that according to the research, the correct option is false, the statement related to a chemical reaction is false.
Learn more about a chemical reaction here: https://brainly.com/question/14929452
#SPJ1
How many grams of carbon would be present in carbon monoxide that contains 2.6 grams of oxygen?
Answers
Answer:
1.95g
Explanation:
Find mass in CO
Find C mass
Using logarithms, you determined the A)Solubility B)Temperatures C)ph
of 7 solutions ranging from
strong acid to neutral to strong base.
Answers
Answer:
the answer is pH
Explanation:
Just took it on ed2020
Answer:
The first part is pH
The second part is calibrate
The third part is all three
Using various concentrations of HCl and NaOH to measure a range of pH
Using an existing scale to determine pH
Knowing that the cabbage indicator always has the same color at a given pH value
Explanation:
Which one of the samples contains the most molecules?
o
A. 1 mol of CO2(g)
B. 1 mol of UF6(g)
O C. 1 mol CH3COOH()
D. They all have the same number of molecules
Answers
Answer: D is right
Explanation: One mole contains 6.0225 ·10^23 molecules
Despite of the substance
How many electrons does the Ca^2+ ion possess
Answers
Answer:
18 electrons
Explanation:
Answer:
18 electrons
Explanation:
Ca has 20 electrons. Ca^2+ has 18 electrons
The steady state vital to life is possible because:________
a. the cell cannot convert energy from one form to another.
b. all cells are autotrophic.
c. all reactions are exothermic.
d. the cell continually takes up energy from the environment.
e. all reactions are at equilibrium.
Answers
Answer: The correct answer is e) all reactions are at equilibrium.
Explanation: In order for cellular vitality to develop, it is necessary for it to be in energetic balance with the environment, that is, to give and receive energy with the environment that surrounds it through endothermic or exothermic reactions. That is why the development of life is considered a system that constantly exchanges with the environment. In turn, that the cell unit maintains a balance with the environment causes homeostasis to occur among the whole organism.
Which of the following pairs of pure substances are written in order of increasing vapor pressure?
I: C2H6 < C4H10
II: NH3 < PH3
III: CH3OCH3 < CH3CH2CH3
Answers
The following pairs of pure substances are written in order of increasing vapor pressure are: I. C₂H₆ < C₄H₁₀.
What is vapor pressure?Vapor pressure is the pressure exerted on a liquid from the vapor of the liquid itself. It is also known as equilibrium vapor pressure or saturation vapor pressure and is the pressure at which a liquid and its vapor are in equilibrium. Vapor pressure is an important factor in determining the state of a liquid. It increases as the temperature increases and decreases as the temperature decreases. It is an intensive property, meaning it is independent of the amount of liquid present.
I: C₂H₆ < C₄H₁₀: This is correct. C₂H₆ has a lower molecular weight and thus a lower vapor pressure than C₄H₁₀.
II: NH₃ < PH₃: This is also correct. NH₃ has a higher molecular weight and thus a higher vapor pressure than PH₃.
III: CH₃OCH₃ < CH₃CH₂CH₃: This is incorrect. CH₃OCH₃ has a lower molecular weight and thus a lower vapor pressure than CH₃CH₂CH₃.
To learn more about vapor pressure
https://brainly.com/question/4463307
#SPJ4
plz answer plz i swear will mark u brainiest

Answers
Answer:
B
Explanation:
sorry if it is wrong
HOW MANY LITERS OF H2 DO YOU HAVE IF YOU START WITH 1.5 MOLES OF H2?
Answers
If you started with 1.5 moles of H2 at STP, you would have approximately 33.6 liters of volume of hydrogen (H₂) gas.
What is the volume of the hydrogen gas at STP?
To determine the number of liters of H2 you have, we need to consider the conditions under which the gas is being held (i.e. temperature and pressure), as well as the molar volume of H2 at those conditions.
At standard temperature and pressure (STP), which is 0°C (273.15 K) and 1 atm (101.325 kPa), the molar volume of any ideal gas is approximately 22.4 L/mol.
Therefore, at STP, 1.5 moles of H₂ would occupy approximately:
V = n x Vm = 1.5 mol x 22.4 L/mol = 33.6 L
Learn more about volume of gas here: https://brainly.com/question/25736513
#SPJ1
The complete question is below:
HOW MANY LITERS OF H2 DO YOU HAVE IF YOU START WITH 1.5 MOLES OF H2? (assume STP condition)
What MASS of NaCl are required to make 2.69L of a 0.14M solution?Use the correct abbreviation for the UNITS
Answers
To solve this problem, let's use the definition for molarity:
Replacing the values of the problem:
Now, to find the mass, we multiply by the molecular weight of NaCl. (Which is about 58.44g/mol)
The answer is approximately 22.2g of NaCl



.Water and toluene are not miscible. Which of the following is most likely the formula of toluene?CH3COOHC6H12O6CH3OHC6H5CH3
Answers
Water and toluene are not miscible, the formula of toluene is (d) C₆H₅CH₃.
What is toluene?Toluene, a substituted aromatic hydrocarbon, is also known as toluol. It is an insoluble in water odorless liquid with the characteristic odor of paint thinners.
It is a phenyl group connected to a methyl group and is a mono-substituted benzene derivative (CH3). Benzene is the parent of the chemical. The IUPAC has referred to it as "methylbenzene" as a result. Toluene is largely used in the industrial sector as a feedstock and a solvent.
Because of their polarity and functional group pressure, the other options are soluble, making option D the correct response.
To know more about Toluene, refer
https://brainly.com/question/8696404
#SPJ4
What is the bulk modulus of oxygen if 34.3 g of oxygen occupies 27.9 l and the speed of sound in the oxygen is 342 m/s?
Answers
The density of oxygen gas is
ρ= 0.0224 m 3
0.0320 kg
=1.43 kg/m 3
From v= B/ρ
we find
B=v 2 ρ
=(317 m/s) 2 (1.43 kg/m 3 )
=1.44×10 5 Pa.
Speed of sound
The speed of sound is the distance travelled per unit of time by a sound wave as it propagates through an elastic medium. At 20 °C (68 °F), the speed of sound in air is about 343 metres per second (1,125 ft/s; 1,235 km/h; 767 mph; 667 km), or one kilometre in 2.9 s or one mile in 4.7 s. It depends strongly on temperature as well as the medium through which a sound wave is propagating. At 0 °C (32 °F), the speed of sound in air is about 331 m/s (1,086 ft/s; 1,192 km/h; 740 mph; 643 km).
To learn more about the speed of sound refer here:
https://brainly.com/question/15381147
#SPJ4
stoichometry in chemistry!! please someone help i'm begging you, if i fail this then i fail chemistry

Answers
Answer:
You can find in the given attachemnet



A machine shop worker records the mass of an aluminum cub as 176 g. If one side of the cube measures 4 cm, what is the density of aluminum?
Answers
Explanation:
Mass=176g
Volume of cube= length ^3
=4 x 4 x 4
=64cm^3
Density = mass/volume
=176/64
=2.75g/cm^3
Determine if the following reaction is a redox reaction. Use evidence from the equation to explain your reasoning.

Answers
A redox reaction is a chemical reaction in which one or more of the reacting species undergoes oxidation and one or more undergoes reduction. An oxidizing agent is an element or compound that oxidizes another substance, while a reducing agent is an element or compound that reduces another substance.
The following reaction is a redox reaction based on the following evidence: 2Al + 3FeO → Al2O3 + 3Fe2+ In this reaction, Fe is being reduced because the FeO is changing to Fe2+. Additionally, the Al is being oxidized because it is losing electrons and forming Al2O3. Therefore, the reaction is a redox reaction. Let us take a look at the oxidation state of the elements in the given equation. Oxidation state of Al: (2) for the reactant and (3+) for the product. Oxidation state of Fe: (2+) for the reactant and (2+) for the product. Oxidation state of O: (-2) for the reactant and (-2) for the product. We can tell that oxidation is happening because of the increase in the oxidation state of Al from 2 to 3+. We can tell that reduction is happening because of the decrease in the oxidation state of Fe from 2+ to 2. As a result, the given equation is a redox reaction.For such more question on oxidizes
https://brainly.com/question/14041413
#SPJ8
A wavelength is defined as the distance from
A. Crest to crest
B. Trough to resting position
C. Crest to trough
D. Crest to resting position
Answers
A.Crest to crest
What amino acid is most famous for its role in the stimulation of human growth hormone, creatine synthesis, and nitric oxide production?
Answers
Answer:
L-Arginine
Explanation:
L-arginine is an essential amino acid that have the effects on growth hormone secretion and nitric oxide production.
A predisposition test is required with toners because they contain which type of derivative? a. aniline b. artifical c. oxygen d. hydrogen,
Answers
A predisposition test is required with toners because they contain which type of derivative is aniline. The correct option is a.
Toners, especially hair toners, often contain derivatives of aniline. Aniline derivatives are commonly used in hair toners due to their ability to help neutralize or counteract unwanted tones in the hair. These derivatives are often referred to as aniline dyes or aniline-based compounds.
Aniline is a chemical compound that belongs to the aromatic amine group. It is commonly used in the production of dyes, pharmaceuticals, rubber additives, and other industrial applications. Aniline derivatives can have different chemical structures and properties, allowing them to interact with hair pigments and modify the hair color.
When using toners that contain aniline derivatives, it is important to perform a predisposition test or patch test. This test involves applying a small amount of the toner on a small patch of skin, usually behind the ear or on the inner forearm, to check for any adverse reactions or allergies. This precautionary step helps ensure the safety and compatibility of the toner with an individual's skin and helps prevent potential allergic reactions.
Therefore, the correct answer is a. aniline, as toners may contain derivatives of aniline that require a predisposition test before use.
Here you can learn more about aniline
https://brainly.com/question/31081529#
#SPJ11
What property of a metal does the image represent
Answers
Answer:
malleable
Explanation:
The image represent in malleable property of metal.
The image possibly represents the photoelectric effect of a metal, which is when it emits electrons after being exposed to electromagnetic radiation. Metals are also characterized by physical properties such as conductivity, malleability, metallic luster, and metallic bonding.
Explanation:Based on your question, the image possibly represents the photoelectric effect, a key property of metals. This phenomenon occurs when a metal surface exposed to electromagnetic waves of a certain frequency absorbs radiation and emits electrons. These emitted electrons are called photoelectrons. Metals can also exhibit free electron model behavior, where electrons freely roam within the metal structure.
Metals possess unique physical properties like conductivity, malleability, and metallic luster. Malleability refers to the metal's ability to deform without breaking, while conductivity refers to the metal's ability to transfer heat or electricity. A metallic luster gives metals their characteristic shiny appearance.
Finally, metals are also known for their metallic bonding—a unique force that holds together the atoms within a metallic solid. Metallic bonding gives rise to many useful and varied bulk properties of metals.
Learn more about Properties of Metals here:https://brainly.com/question/33514448
#SPJ2
Please help
in a test rn so id be greatly appreciated if u answered quickly!!

Answers
Answer:
Its the second answer.
Explanation:
Answer:
my best guess is B or C. first and last don't make sense. sorry i can't give u a straight answer lol
Explanation:
1. What is the mass of 550 mL of air at atmospheric pressure?
Answers
Answer:
0.715 g
Explanation:
mass = 0.0013x550= 0.715 g
HELP PLEASE
all charged objects create an electric field around them. what two factors determine the strength of two electric fields upon the charged objects creating them?
1) how charged the object creating the field is and the density of the charged objects
2) how charged the object creating the field is and weather the objects are positively or negatively charged
3) how charged the object creating the field is and the mass of the two charged objects
4) how charged the object creating the field is and the distance between the two charged objects
Answers
Answer:
4) how charged the object creating the field is and the distance between the two charged objects
Sodium hydroxide solution reacts with copper (II) nitrate solution to produce a
a. White precipitate
b. Colourless precipitate
c. Blue precipitate
d. Brown precipitate
Answers
Answer:
brown precipitate.. d.. option
what is the name of CCI3
carbon trichlorine????
Answers
Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVACR) is an organic compound with the chemical formula CCl4.
How many formula units are in 0.25 mole of NaCl?
Answers
Answer:
The meaning and usefulness of the mole Page 2 2 • One mole of NaCl contains 6.022 x 1023 NaCl formula units. Use the mole quantity to count formulas by weighing them. The mass of an atom in amu is numerically the same as the mass of one mole of atoms of the element in grams.
Explanation:
Formula Units = mole x Avogadro's Number
Formula Units of NaCl = mole NaCl x Avogadro's Number
Formula Units of NaCl = 0,25 mole x 6,022 x 10²³ molecule
Formula Units of NaCl = 1,5055 x 10²³ molecule
please HELP!! will mark brainiest

Answers
What is the subscript charge?
Answer:
Lose 3
Explanation:
Aluminum has a full outer shall, ergo, when it forms an ion it can only lose.
Type of Government
Example
Leader/Info
Communist State
Presidential Republic
Parliamentary Republic
Constitutional Monarchy (with ceremonial monarch)
Constitutional Monarchy (with active monarch)
Absolute Monarchy
Theocracy
Federal System
Unitary System

Answers
The following prompt looks at various kinds of governments and the countries practicing them. See the attached image. One example is India tha practices the Parliamentary Republic.
What is a parliamentary republic?A parliamentary republic is a republic that works under a parliamentary form of government in which the executive branch is legitimated by and accountable to the legislature. There are several types of parliamentary republics.
Alabnia, Armenia, Austria, Bangladesh, Barbados, Bosnia and Herzegovina, Botswana, Bulgaria, Croatia, Czech Republic, Dominica, Estonia, Ethiopia, Fiji, Finland, Georgia, Germany, Greece, Guyana, Hungary, Iceland, India, Iraq, Ireland, and others are parliamentary republics.
Learn more about Parliamentary Republic;
https://brainly.com/question/3710037
#SPJ1