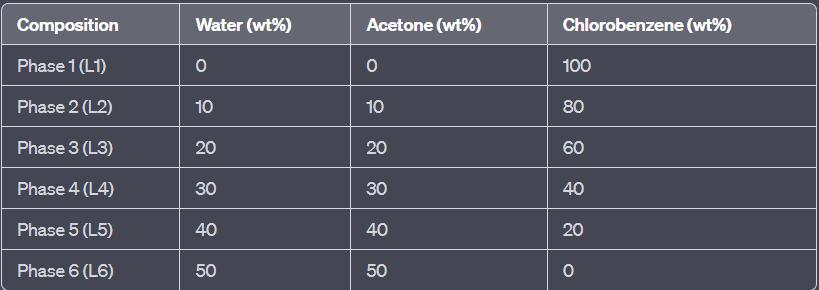
For the water + acetone + chlorobenzene system, construct the equilibrium diagram. Experimental data is shown in the table below. Plot the binodal curve, the critical point and the conjugation line eq
Answers
The equilibrium diagram for the water + acetone + chlorobenzene system includes the binodal curve, the critical point, and the conjugation line.
To construct the equilibrium diagram, we need experimental data, which is shown in the table attached below.
Now let's plot the equilibrium diagram:
Binodal curve:
The binodal curve represents the boundary between the liquid-liquid immiscibility region and the single-phase region. To plot the binodal curve, we connect the points corresponding to the compositions of the phases.
Critical point:
The critical point represents the highest temperature and pressure at which a liquid-liquid immiscible system can exist. To determine the critical point, we need additional experimental data, including temperature and pressure values for each composition.
Please provide the temperature and pressure values for the experimental data, or specify if they are not available.
Conjugation line:
The conjugation line represents the boundary between the liquid-liquid immiscibility region and the liquid-vapor immiscibility region. It is determined by finding the compositions where the phases exhibit the maximum difference in boiling points.
Once again, we need additional data, specifically the boiling points of the mixtures at each composition. Please provide the boiling point data or specify if it is not available.
To construct the equilibrium diagram for the water + acetone + chlorobenzene system, we require additional information such as temperature, pressure, and boiling point data.
Once we have this data, we can plot the binodal curve, critical point, and conjugation line, providing a comprehensive representation of the system's phase behavior.
For the water + acetone + chlorobenzene system, construct the equilibrium diagram. Experimental data is shown in the table below. Plot the binodal curve, the critical point and the conjugation line equilibrium concentration of the coexisting phases (mass fraction) aqueous phase organic phase water acetone chlorbenzene water acetone chlorbenzene 0.9989 (0) 0.0011 0.0018 0 0.9982 0.8979 0.1 0.0021 0.0049 0.1079 0.8872 0.7969 0.2 0.0031 0.0079 0.2223 0.7698 0.6942 0.3 0.0058 0.0172 0.3748 0.608 0.5864 0.4 0.0136 0.0305 0.4944 0.4751 0.4628 0.5 0.0372 0.0724 0.5919 0.3357 0.2741 0.6 0.1259 0.2285 0.6107 0.1608 0.2566 0.6058 0.1376 0.2566 0.6058 0.1376
To learn more about equilibrium, visit
https://brainly.com/question/30772553
#SPJ11
Related Questions
Please help!!! If u reply with a link i will report
M = mRT/PV
Use the equation above to find the molar mass of a 98.2 g sample of gas that fills a 50.0 liter container at STP
a. 4.00 g/mol
b. 44.0 g/mol
c. 1.48 g/mol
d. 32.0 g/mol
Answers
the half life of a radioactive substance is 1459 years. what is the annual decay rate? express the percent to 4 significant digits.
Answers
The relation between half-life and rate at which it is decaying is given by
t(1/2) = log2/rate
now in the above problem half-life is given as 1459 years substituting in the above equation we get the value of rate at which it is decaying to be rate=0.0001075.
The amount of substance that is present after t years given that the half-life of the substance is h is calculated through the equation,
A(t) = A(o)(0.5^t/h)
The 1/2-life of a radioactive isotope is the quantity of time it takes for one-1/2 of the radioactive isotope to decay. The half of-existence of a selected radioactive isotope is consistent; it's miles unaffected by using situations and is independent of the initial quantity of that isotope.
1/2-life (symbol t1⁄2) is the time required for an amount (of substance) to reduce to half of its initial price. The time period is commonly utilized in nuclear physics to explain how speedy volatile atoms undergo radioactive decay or how long strong atoms survive.
To know more about decay, refer: https://brainly.com/question/20341401
#SPJ4
people tint their car windows to stop what

Answers
Answer:
Reflection
Explanation:
Answer:
b
Explanation:
Determine the coordination number for each structure.
a. Gold
b. Ruthenium
c. Chromium
Answers
A person with tuberculosis is given a chest x-ray. Four tuberculosis x-ray specialists examine each x-ray independently. If each specialist can detect tuberculosis 79% of the time when it is present, what is the probability that at least 1 of the specialists will detect tuberculosis in this person? P( at least 1 specialist detects tuberculosis )= (Round to four decimal places as needed.)
Answers
The probability that at least one of the specialists will detect tuberculosis in this person is 0.9994.
Given that a person with tuberculosis is given a chest x-ray. Four tuberculosis x-ray specialists examine each x-ray independently. If each specialist can detect tuberculosis 79% of the time when it is present.The probability that at least 1 of the specialists will detect tuberculosis in this person is to be calculated.
P( at least 1 specialist detects tuberculosis )=?
The probability that each specialist can detect tuberculosis = P(Detecting tuberculosis) = 79/100 = 0.79
The probability that the specialist cannot detect tuberculosis = P(Not detecting tuberculosis) = 1 - P(Detecting tuberculosis) = 1 - 0.79 = 0.21
Let A be the event that the specialist can detect tuberculosis.
Let B be the event that the specialist cannot detect tuberculosis.
Then, P(A) = 0.79, and P(B) = 0.21
Now, we need to find the probability that at least one of the specialist detects tuberculosis.The probability that at least one of the specialist detects tuberculosis is given as :
P(at least one of the specialist detects tuberculosis) = 1 - P(no specialist detects tuberculosis)
P(no specialist detects tuberculosis) = P(Not detecting tuberculosis) for the 1st specialist × P(Not detecting tuberculosis) for the 2nd specialist × P(Not detecting tuberculosis) for the 3rd specialist × P(Not detecting tuberculosis) for the 4th specialist = 0.21 × 0.21 × 0.21 × 0.21 = (0.21)^4
Putting this value in the formula :
P(at least one of the specialist detects tuberculosis) = 1 - P(no specialist detects tuberculosis)
= 1 - (0.21)^4 = 0.9994= 0.9994 (rounded to four decimal places)
Therefore, the probability is 0.9994.
To learn more about tuberculosis :
https://brainly.com/question/18173152
#SPJ11
How many atoms are present in 4.56moles of sulfur?
Answers
Avogadro's number (6.02*1023) of something is defined as 1 mole. So one mole of sulfur (or any other element) equals 6.02*1023 atoms. 4.56 moles is only 4.56 times as much.
What is atoms?An atom is a matter particle that defines a chemical element uniquely. An atom is made up of a central nucleus and one or more negatively charged electrons. The nucleus is positively charged and contains one or more heavy particles called protons and neutrons. An atom is a fundamental particle of matter that contains at least one proton. Here are a few atom examples: neon (N) and hydrogen (H) (Ne). Because atoms were once thought to be the smallest things in the universe and could not be divided, the term "atom" comes from the Greek word for indivisible.To learn more about atoms, refer to:
https://brainly.com/question/6258301
#SPJ4
please solve this problem. IT IS TOO URGENT ♂️

Answers
Answer:
1. Sulphuric acid
2. Car battery acid
3. Washing up liquid
4. Milk of magnesia
5. Metal polish
6. Oven cleaner
Explanation:
Universal paper can determine the pH of a solution. It ranges from dark red (pH 0 - very acidic) to orange/yellow, to green (pH 7 - neutral) to blue, dark blue and purple (pH 14 - very alkaline)
Sulphuric acid has the lowest pH as the universal indicator is red.
The next is car battery acid which is pink - not as acidic as red.
The next is washing up liquid, which is yellow, around pH 3 or 4.
The next is milk of magnesia with light blue, around pH 9 or 10.
Metal polish is dark blue - around pH 11.
Oven cleaner is the darkest, with purple.
provide an acceptable name of the compound below. spell out the full name of the compound.
Answers
The compound is called 2,4-dimethylpentane.
What is the full name of the compound?2,4-dimethylpentane is a hydrocarbon compound consisting of five carbon atoms arranged in a linear chain with two methyl groups attached to the second and fourth carbon atoms. The prefix "2,4-dimethyl" indicates the positions of the methyl groups, while "pentane" signifies the presence of a five-carbon chain. This compound belongs to the alkane family, which is characterized by single bonds between carbon atoms and saturated hydrocarbon structures.
2,4-dimethylpentane is an organic compound commonly used as a solvent in various industries, including pharmaceuticals, paints, and coatings. Its unique molecular structure and chemical properties make it an effective choice for dissolving nonpolar substances. It is a clear liquid with a strong hydrocarbon odor and is highly flammable.
Learn more about Compound
brainly.com/question/14117795
#SPJ11
the general equation for a double-displacement reaction is
Answers
The general equation for a double-displacement reaction is
A + BX ---> AX + B or AX + BY ---> AY + BX
Here, A and B represent two different chemical species (such as ions or molecules), and X and Y represent their respective chemical partners.
The positively charged species (A and B) exchange partners (X and Y) in a double displacement process to create new compounds (AX and BY). Because the positively charged species "switch" partners, this kind of reaction is often referred to as a "swap" reaction.
Double-displacement reactions include the following examples:
1. The transformation of silver nitrate (AgNO3) into sodium nitrate (NaNO3) by the reaction of sodium chloride (NaCl) and AgNO3:-
NaCl(aq) + AgNO3(aq) AgCl(s) + NaNO3(aq)
2. The transformation of barium chloride (BaCl2) into barium sulfate (BaSO4) and sodium chloride (NaCl) via the following equation:-
BaCl2(aq) + Na2SO4(aq) BaSO4(s) + 2NaCl(aq)
Depending on the chemical makeup of the reactants, not all double-displacement reactions are metathesis reactions, it is crucial to keep in mind.
To know more about double-displacement reaction please refer: https://brainly.com/question/29307794
#SPJ4
A sinkhole is a depression that forms in the ground as the surface layer slowly or quickly collapses. Rainwater is naturally a weak acid.
Most of the acid in the rainwater is carbonic acid which reacts with limestone and changes it into a new substance. The new substance
dissolves in the water which drains away. This leaves cracks and holes underneath the surface that can collapse. The formation of one
type of sinkhole is shown below.
topsoil
limestone
"hain
rain
pond
Rainwater dissolves some
limestones
and carries some
of the new solution away. This
forms cracks which results in
a small depression
The dissolving of the limestone
makes bigger cracks. The cracks
are fitted with topsoil and a pond
forms
3
Underneath a sinkhole, cracks can lead to other areas where the limestone has been dissolved. A much larger hole is formed by
the same processes of chemical weathering and erosion. What does this most likely form?
O A. a delta
B.
a lake
C.
a cave
D.
a coastline
Answers
Answer:
b
Explanation:
I'm guessing don't hold me to it
Answer: 3. C) a cave
Explanation:
A sinkhole is a hole in the ground caused by the decline of water levels and disturbance of the soil. If you'd like to imagine how it looks, search "sinkhole".
If a sinkhole occurs, more cracks form and the hole itself becomes larger, what do you expect would happen?
The formation could become a cave.
The answer is C. A cave.
Hope this helps!
Even before modern observations provided evidence supporting the theory of plate tectonics, people developed theories that the continents were once joined together. Using only maps, what did they observe?
Answers
Answer:
people or scientist tend to observe the nature of the continents that described by jigsaw fit,geological similarity among the continents
please help with this need it in 6 hours
1.)in the thermit reaction 2Al + Fe2o3= al2o3 + 2 fe. what is the mass of aluminium powder is needed to react with 8.0g of iron (3) oxide
2.) what mass of magnesium sulphate crystals can be made from14.0g of magnesium carbonate and an excess of dilute sulphuric acid
3.) what mass of calcium oxide is formed when 25g of calcium carbonate is decomposed by heat?
4.) Lead (ii) oxide PbO, reacts with hydrogen to for lead and steam. calculate the mass of lead in formed when 446g of lead (ii) oxide is reduced in this way.
5.) what volume of gas is given off when excess calcium carbonate is added to 50cm cube of 2.0M by hydrochloric acid , if the gas volume is measured at s.t.p.?
Answers
1) Mass of aluminum powder is 2.7 g.
2) Mass of magnesium sulfate is 19.9 g.
3) Mass of calcium oxide is 14g.
4) Mass of lead 412.2 g.
1) In the thermite reaction :
2Al + Fe₂O₃ -----> Al₂O₃ + 2Fe
mass of Fe₂O₃ = 8.0 g
molar mass of Fe₂O₃ = 159.6 g/mol
number of moles = mass / molar mass
= 8.0 g / 159.6 g/mol
= 0.050 moles
1 mole of Fe₂O₃ react with 2 mole of Al ,
0.050 Fe₂O₃ moles react with 2 × 0.050 = 0.1moles of Al
number of moles of Al = mass / molar mass
mass of Al = no. of moles × molar mass
= 0.1 mol × 27 g/mol
= 2.7 g
Thus, the mass of aluminum powder is needed to react with 8.0 g of iron (III) oxide is 2.7 g
2) the reaction is :
MgCO₃ + H₂SO₄ -----> MgSO₄ + CO₂ + H₂O
mass of MgCO₃ = 14.0 g
no. of moles of MgCO₃ = mass / molar mass
= 14 g / 84.3 g/mol
= 0.166 moles
1 mole of MgCO₃ produced 1 mole of MgSO₄,
0.166 mole of MgCO₃ produced 0.166 mole of MgSO₄
mass of MgSO₄ = 120.3 g/mol
no. of moles of MgSO₄ = mass / molar mass
mass of MgSO₄ = no. of moles × molar mass
= 0.166 mol × 120.3 g/mol
= 19.9 g
Thus, mass of magnesium sulfate crystals can be made from14.0g of magnesium carbonate and an excess of dilute sulfuric acid is 19.9 g
3) the reaction is :
CaCO₃ -----> CaO + CO₂
mass of calcium carbonate = 25 g
molar mass of CaCO₃ = 100 g/mol
no. of moles of = mass / molar mass
= 25 / 100
= 0.25 mol
0.25 moles of CaCO₃ produced 0.25 moles of CaO
molar mass of CaO = 56 g/mol
mass of CaO = no. of moles × molar mass
= 0.25mol × 56 g/mol
= 14 g
the mass of CaO produced 14 g.
4) PbO + H -----> Pb + H₂O
mass of PbO = 446g
no. of moles = mass / molar mass
= 446 g / 223.3 g/mol
= 1.99 mols
1.99 moles of PbO produced 1.99 moles of Pb
molar mass of Pb = 207.2 g/mol
mass of Pb = no. of moles × molar mass
= 1.99 mol × 207.2 g/mol
= 412.2 g
Thus, Lead (ii) oxide PbO, reacts with hydrogen to for lead and steam. calculate the mass of lead in formed when 446g of lead (ii) oxide is 412.2g.
To learn more about no. of moles here
https://brainly.com/question/26416088
#SPJ1
is sugar ionic or covalent
Answers
The sugar is the covalent compound. The sugar is composed of the carbon, the oxygen, and the hydrogen .
The Sugar, are composed of the carbon, the oxygen, and the hydrogen and has the covalent bonds. The covalent bond is formed when the bond is formed by the mutual sharing of the electrons in between the atoms or the molecules.
The compound formed by the covalent bond is called as the covalent compounds. Therefore, the sugar is the covalent compound as all the covalent bonds in the sugar molecules arise by the result of the electron sharing in between the atoms.
To learn more about covalent here
https://brainly.com/question/30420584
#SPJ4
Typically, when two atoms form a chemical bond, the expected result is that the electrons.
Answers
When atoms form chemical bonding then the atoms attain Noble gas configuration.
Noble gas configuration of an atom includes the fundamental image of the ultimate noble fueloline previous to that atom, observed via way of means of the configuration of the ultimate electrons.so for sodium, we make the substitution of [Ne] for the 1s22s22p6 a part of the configuration. Sodium's noble fueloline configuration becomes [Ne]3s1.
Covalent bonds, atoms percentage pairs of electrons, at the same time as in ionic bonds, electrons are absolutely transferred among atoms in order that ions are formed.During the formation of a chemical bond, the predicted end result is that the electrons will whole a noble fueloline configuration in each atoms. Typically, while atoms shape a chemical bond, the predicted end result is that the electrons.
Read more about atom:
https://brainly.com/question/621740
#SPJ4
What is the "major drawback" to using word equations?
a) Word equations can't be translated to other
languages.
b) Words are not specific enough to identify
chemicals.
C) Word equations are not quantitative.
Answers
Answer:
C
Explanation:
they can be put in other languages
they are literally the name of the chemical
they don't show quantity
The "major drawback" to using word equations is that they are not quantitative. Option C is correct.
Chemical reactions are written in word equations to give a clear and concise understanding of the reaction taking place. However, word equations are not quantitative. A equation is a way of representing a chemical reaction using words. It identifies the reactants and products and their physical states as well as the condition under which the reaction is taking place.
For example, the word equation for the reaction between hydrogen gas and oxygen gas to produce water is:
Hydrogen gas + oxygen gas → water
Quantitative equations use chemical formulas, numbers and symbols to represent the reactants and products in a chemical reaction. They provide a quantitative understanding of the reaction in terms of the number of atoms and molecules involved.
Know more about equations:
https://brainly.com/question/29657983
#SPJ6
You have 2.2 mol Xe and 2.0 mol F₂, but when you carry out the reaction you end up with only 0.25 mol XeF₄. What is the percent yield of this experiment? Xe(g) + 2 F₂ (g) → XeF₄ (g)
Answers
Answer:
The correct answer is 25 %
Explanation:
According to the chemical reaction:
Xe(g) + 2 F₂ (g) → XeF₄ (g)
1 mol of Xe(g) reacts with 2 mol of F₂(g), so the stoichiometric ratio os reactants is 2 mol F₂/mol Xe.
We have 2.2 mol Xe and 2.0 mol F₂, so the ratio is:
2.0 mol F₂/2.2 mol Xe = 0.909 mol F₂/mol Xe
The molar ratio of reactant we have is lower than the required, so F₂ is the limiting reactant.
By using the limiting reactant, we calculate the theoretical amount of product (XeF₄). For this, we know that 1 mol of XeF₄ is formed from 2 mol of F₂ (1 mol XeF₄/ 2 mol F₂), and we have 2.0 mol F₂:
2.0 mol F₂ x (1 mol XeF₄/ 2 mol F₂)= 1 mol XeF₄
If we only obtained 0.25 mol XeF₄, the percent yield of the experiment is:
Yield = experimental amount/theoretical amount x 100%
= 0.25 mol XeF₄/ 1 mol XeF₄ x 100% = 25 %
The percent yield of the solution is 25%.
The equation of the reaction is;
Xe(g) + 2F₂ (g) → XeF₄ (g)
From the reaction equation;
1 mole of Xe reacts with 2 moles of F2
2.2 moles of Xe will react with 2.2 moles × 2 moles /2 moles
= 4.4 moles of F2
We can see that there is not enough F2 hence it is the limiting reactant
2 moles of F2 yields 1 mole of XeF4
2 moles of F2 yields 2 moles × 1 mole/2 moles = 1 mole of XeF4
% yield = actual yield/Theoretical yield × 100
% yield = 0.25 mole/ 1mole × 100
% yield = 25%
Learn more: https://brainly.com/question/2510654
A scientist wants to investigate the effect of concentration of nutrient agar to the growth of bacteria. He created 3 set ups: A) 30 g nutrient agar powder in 1 L H2O B) 40 g nutrient agar power in 1L H2O C) 50 g nutrient agar powder in 1 L H2O
1) Based from the given scenario, CREATE a testable hypothesis using IF-THEN- BECAUSE statements.
2) Using your hypothesis, IDENTIFY the dependent and the independent variables.
Answers
what method will you use to separate the mixture of two solids which have different solubility in water
Answers
Answer:
A fractional crystallisation method is used for separating a mixture of two solids, if their solubilities in a particular solvent differ widely.
Explanation:
Could someone please help me with this?

Answers
B. The final speed of the ball when it hits the ground is determined as 24.2 m/s.
Final speed of the ballThe final speed of the ball can be determined by applying the principle of conservation of energy as follows;
K.E = P.E
¹/₂mv² = mgh
v² = 2gh
v = √2gh
where;
v is the maximum velocity of the ballh is the initial height of the ballg is acceleration due to gravitySubstitute the given parameters and solve for the maximum speed of the ball.
v = √(2 x 9.8 x 30)
v = 24.2 m/s
Thus, the final speed of the ball when it hits the ground is determined as 24.2 m/s.
Learn more about final speed here: https://brainly.com/question/6504879
#SPJ1
Help I’ll give brainlist

Answers
Answer:
A, And D
Explanation:
I did this before and i got it right ❤
1. When frequency increases energy:
a) Increase and wavelength increases
b) Increases and wavelength decreases
c) decreases and wavelength decreases
d) decreases and wavelength increases
Answers
When frequency increases, as does the energy, but wavelength decreases. It also works vise versa; if wavelength were to increase, its frequency and energy will decrease.
Show all your work and make sure to label all the numbers with the correct units if you do, I will give you brainly:
A: A sample of sulfuric acid has a mass of 15.0 g. How many molecules are in this sample?
B: How many moles of sulfuric acid are present in 45 g of this sample?
C: How much would a .750 mole sample of Ammonium sulfate weigh?
D: What is the mass percent of hydrogen in this sample?
Answers
A = 21 molecules
B = 0.45 moles
C = 99.11475 grams
D = 2.055%
Explanations are below
1. Write the formula for sulfuric acid
H2SO4
2. Find how many g/mol sulfuric acid is
H = 2*1.008 = 2. 016
S = 1 * 32.07 = 32.07
O = 4 * 15.999 = 63.996
Add the products together
2.016 + 32.07 + 63.996 = 98.082 g/mol
2. Convert grams to mol
15.0 grams * 1 mol/98.082 = 0.152933259925369 ~ 0.15 mol
3. Convert mol to molecules
0.15 mol * 6.02*10^23 molecules/ 1 mol = 20.769 molecules ~ 21 molecules
B.
1. Convert grams to moles
45 grams * 1 mol/ 98.082 grams = 0.458799779776106 ~ 0.45 moles
C.
1. Write the formula for ammonium sulfate
(NH₄)₂SO₄
2. Find the weight of the formula above
N = 2 * 14.01 = 28.02
H = 8 * 1.008 = 8.064
S = 1 * 32.07 = 32.07
O = 4 * 15.999 = 63.996
Add the products together
28.02+8.064+32.07+63.99 = 132.153 g/mol
3. Convert mol to grams
0.750 mol * 132.153 g/ 1 mol = 99.11475 grams
D.
1. Find the molar mass of all elements and combined
H = 2*1.008 = 2. 016
S = 1 * 32.07 = 32.07
O = 4 * 15.999 = 63.996
Add the products together
2.016 + 32.07 + 63.996 = 98.082 g/mol
2. To find the mass percent, use the equation molar of element divided by molar mass of the products combined in parentheses times 100.
(2.016/98.082) * 100 —> 0.02055423013397 * 100 = 2.05542301339695 %
This can written as 2.055% since 2.016 has 4 significant figures
how do you prepare 4.5L solution of 1.5M NaCl
Answers
Answer:
mol = 6.75
Explanation:
Remember the equation for Molarity:
Molarity = mol/liters
So,
1.5M = mol/4.5
not isolate mol by multiplying 4.5 by both sides, using algebra
mol = 6.75
A gymnast whose weight is 5 10 N hangs from the middle of a bar supported by two vertical strands of rope. What is the tension in each strand?
A. 765 N
B. 0 N
C. 1020 N
D. 510 N
E. 255 N
Answers
5. Both kerosene and water are liquids at room temperature. Describe a procedure that can be used
to separate a mixture of the two liquids.
Answers
I found this. hope it helps!

Do you think that participation in a Supervised Agriculture Experience (SAE) prepares a student for a career in the AFN industry? Discuss why you agree or why you don’t. Discuss how your particular SAE has prepared you for your future career.
HELPPPP !! For horticulture
Answers
Answer:
The SAE curriculum includes practical farming tasks conducted outside the scheduled classroom and laboratory period by students. SAEs offer a method for students in agricultural education to gain real-world work opportunities that they are most interested in in the field of agriculture. Supervised agricultural experience is an essential component of agricultural education, and all Agriculture, Food and Natural Resources (AFNR) courses are a necessary component.
Explanation: Hope it helps
Which statements about β turns are correct? Their purpose is to reverse the direction of the polypeptide chain. There are two types, I and II, which differ mainly in the conformation about the i+1 and i+2 residue amide bond. They typically contain large, hydrophobic residues. Their conformation is held in place through H bonds.
Answers
Answer:
The correct answer is - 1, 2, and 4 statements.
Explanation:
Beta and gamma turns are common plots or turns in proteins and contain intra-turn hydrogen bonds. This hydrogen bond is present between CO of residue i and NH of residue i+3 that holds the confirmation in beta turns.
Beta turns, assist the protein to get their globularity, as the aim of beta turns is to reverse the direction of the polypeptide. The two main of beta turns are type-I and type-Il. and their minor images are type-I and type-Il.
Thus, the correct answer is - 1, 2, and 4 statements.
Based on the two starting amounts of Cu and HNO₃, the amount of NO₂ produced should be 0.0944 moles.
Calculate the concentration (in molarity) of the gas if that gas were confined to the 250 mL of space in the flask.
Answers
The concentration (in molarity) of the gas if that gas were sealed to the 250 mL of space in the flask is 0.3776 M.
How do we calculate the molarity?Molarity of any substance is defined as the number of moles of solute present in per liter of the solution and it will be represented as:
M = n/V, where
n = moles = 0.0944mol
V = volume = 250mL = 0.25L
On putting values, we get
M = 0.0944 / 0.25 = 0.3776 M
Hence required molarity is 0.3776 M.
To know more about molarity, visit the below link:
https://brainly.com/question/22283918
#SPJ1
FILL IN THE BLANK. Indicate how many stereoisomers are possible for each compound. a) square planar [Pt(NH3)2Cl2] number ____ stereoisomer(s) b) tetrahedral [NiClBr3]²- number ______ stereoisomer(s) c) octahedral [PtBr4Cl2]²- number _____ stereoisomer(s)
Answers
The number of stereoisomers possible for each compound is:
a) square planar [Pt(NH3)2Cl2] has 1 stereoisomers.
b) tetrahedral [NiClBr3]²- has 2 stereoisomers.
c) octahedral [PtBr4Cl2]²- has 3 stereoisomers.
a) For the square planar compound [Pt(NH3)2Cl2], there is only 1 stereoisomer possible. This is because the arrangement of ligands in a square planar geometry does not produce any distinct spatial orientations that could be considered as different stereoisomers.
b) For the tetrahedral compound [NiClBr3]²-, there are 2 stereoisomers possible. These are the enantiomers (mirror image isomers) due to the presence of a single central atom with four different ligands attached in a tetrahedral arrangement.
c) For the octahedral compound [PtBr4Cl2]²-, there are 3 stereoisomers possible. These include 1 cis isomer and 2 trans isomers, as the octahedral geometry allows for distinct spatial arrangements of the ligands with respect to each other.
In summary, [Pt(NH3)2Cl2] has 1 stereoisomer, [NiClBr3]²- has 2 stereoisomers, and [PtBr4Cl2]²- has 3 stereoisomers.
To know something about the stereoisomers, click below.
https://brainly.com/question/31862213
#SPJ11
Is oxygen a metal or a nonmetal? how many valence electrons does an oxygen atom have?
Answers
Oxygen is a nonmetal and it has 6 valence electrons. (2s subshell has two and 2p subshell has four)
why oxygen is not a metal ?The element's size shrinks as it moves from left to right in a period, while its electronegativity rises. Therefore, we can conclude that an element's non-metallic property grows as an element moves from left to right over time.Elements get bigger and have lower electronegativities as they move down the group. As a result, their ionization energy drops and they become more metallic.Therefore, if we look at oxygen, it is situated in the sixteenth group, which is to the right of the periodic table, and the second period, which is smaller in size than other elements.
As a result, we can infer that the oxygen atom will have a significant non-metallic character because of its tiny size and strong electronegativity.
what do you mean by valence electron ?The electrons in an atom's outermost shell are called valence electrons. Because the electrons in the outermost shells of two atoms are the first to come into contact with one another and are the ones that control how an atom will react in a chemical reaction, when two atoms interact.
learn more about oxygen atom visit :
https://brainly.com/question/19532510
#SPJ4
Answer:
Oxygen is a non metal, and has 6 valence electrons.
Explanation:
I did it on plato