For the reaction:CO(g) + 2 H2(g) → CH3OH(g) K = 91.4 at 350 K and K = 2.05 x 10-4 at 298K What is the value of ΔHºrxn? Select one: a. 2.08 x 103 kJ/mol b. 217 kJ/mol c. 49.9 kJ/mol d. 446 kJ/mol e. 3.75 x 10-2 kJ/mol
Answers
To determine the value of ΔHºrxn (the standard enthalpy change for the reaction), we can use the equation:
ΔGº = -RT ln(K)
where ΔGº is the standard Gibbs free energy change, R is the gas constant (8.314 J/(mol·K)), T is the temperature in Kelvin, and K is the equilibrium constant for the reaction.
First, let's calculate ΔGº at each temperature using the given equilibrium constants:
At 350 K:
ΔGº(350 K) = -RT ln(K) = -(8.314 J/(mol·K) * 350 K) * ln(91.4) = -2.08 x 10^3 J/mol
At 298 K:
ΔGº(298 K) = -RT ln(K) = -(8.314 J/(mol·K) * 298 K) * ln(2.05 x 10^-4) = 446 J/mol
To calculate ΔHºrxn, we can use the relationship between ΔGº and ΔHº at constant temperature:
ΔGº = ΔHº - TΔSº
where ΔSº is the standard entropy change for the reaction.
By rearranging the equation, we can solve for ΔHº:
ΔHº = ΔGº + TΔSº
At 350 K:
ΔHº(350 K) = -2.08 x 10^3 J/mol + (350 K * ΔSº)
At 298 K:
ΔHº(298 K) = 446 J/mol + (298 K * ΔSº)
Since ΔHº is independent of temperature, we can equate the two equations:
-2.08 x 10^3 J/mol + (350 K * ΔSº) = 446 J/mol + (298 K * ΔSº)
Simplifying the equation:
52 K * ΔSº = 2.526 x 10^3 J/mol
ΔSº = (2.526 x 10^3 J/mol) / 52 K
ΔSº = 48.58 J/(mol·K)
Finally, we can calculate ΔHºrxn using either of the original equations:
ΔHºrxn = ΔHº(350 K) = -2.08 x 10^3 J/mol + (350 K * 48.58 J/(mol·K))
ΔHºrxn ≈ 2.08 x 10^3 J/mol
Therefore, the value of ΔHºrxn is approximately 2.08 x 10^3 J/mol, which is equivalent to 2.08 x 10^3 kJ/mol (option a).
Learn more about reaction Visit : brainly.com/question/11231920
#SPJ11
Related Questions
What is the morality of a solution that contains 80.0 G Al2 (SO4)3 (aluminum sulfate) in 625 g H2O?
Answers
Answers
Explanations
what volume of 12.0 M HCl contains 0.6 mole of HCl? Pls help!!!
Answers
Answer:
volume required = 50 mL
Explanation:
The concentration of a substance is the quantity of solute present in a given quantity of solution.
In an aqueous solution of hydrochloric acid, the concentration is the number of moles of HCl present in a certain volume (which we are calculating) of solution.
we can write an expression for this:
c = n/V, where c = concentration, n = moles, and V = volume in L.
Rearranging this formula, we get:
V = n/c.
Therefore, V = 0.6 / 12.0 = 0.05 L or 50 mL
oxidation involves which of the following? 1. loss of electron(s). 2. gain of electron(s). 3. increase in oxidation state. group of answer choices 2 only 1 only 3 only 1 and 3 only 2 and 3 only
Answers
Electron(s) are lost during oxidation, and the oxidation state is raised.
While reduction includes the acquisition of electrons, oxidation entails the loss of electrons.
This is in line with how oxidation and reduction are defined.
Redox reaction refers to any process in which oxidation and reduction take place simultaneously. When reactants are transformed into products in an oxidation-reduction (redox) reaction, electrons are transferred from one element to another. The change in oxidation values from the reactant side to the product side indicates the transfer of electrons.
As a result, choice D is the right response.
To learn more about oxidation, refer:-
https://brainly.com/question/9496279
#SPJ4
An ecosystem where all organisms have niches and habitats will be
balanced.
unbalanced.
Answers
Answer:
balenced.
Explanation:
An ecosystem where all organisms have niches and habitats will be balanced.
What is an ecosystem ?An ecosystem consists of all the organisms and the physical environment with which they interact.
What is a niche ?Niche describes the role an organism plays in a community. A species' niche encompasses both the physical and environmental conditions it requires and the interactions it has with other species.
What is a habitat ?Habitat is the area and resources used by a particular species.
A balanced ecosystem represents a sustainable habitat of interdependent animals, plants, and microorganisms and their environment.
To know more about ecosystem here
https://brainly.com/question/1061425
#SPJ2
What’s the capital city of Turkey?
Answers
Answer:
Ankara is the capital of Turkey! Hope this helped you out! :)
Explanation:
The capital of Turkey is Ankara
hopefully this helped :3
Since the speed of light, “c”, is 0.3% slower in air than it is in outer space, how does that affect the observed wavelengths?
Answers
Answer:
....interesting
five structural isomers, or constitutional isomers, have the formula c6h14 . draw the indicated isomers, grouped by number of carbon atoms in the main chain.
Answers
The first component is a six-carbon linear structure. Then we'll need to add a methyl group to a five-carbon linear structure.
Constitutional isomers:
This methyl group can be linked to either carbon 2 or carbon 3 in the carbon chain. Finally, we can make a four-carbon linear structure by adding two methyl groups. This addition can be done with carbon 2 alone or with carbon 2 and carbon 3 together.
The constitutional isomers have the same chemical formula, but their atom connections are different. Chain isomers, position isomers, and functional group isomers are the three forms of constitutional isomers.
Find out more about constitutional isomers visit
brainly.com/question/9972986
#SPJ4

20 points for answer: D=m/v. Mass=30g and Volume=6mL
Answers
Answer:
density = 5 g/ml
Explanation:
D=m/v. Mass=30g and Volume=6mL
SO solve for D when m = 30 and v = 6
D=m/v
D = 30/6
density = 5 g/ml
You have available the following ingredients. Which one or ones could you use to make a pH=3 buffer? 1.5MKOH(aq) 3.0MHCl(aq) 1.0MNH 3(aq) 2.5MCH 3COOH(aq) 2.0MKHCOO(aq) 0.5MKCl(aq) Partially correct. The first step is to identify the conjugate acid/base pair that best matches the intended pH. Remember to write of If you only have one (weak acid or weak base) how do you make a solution that has both?
Answers
To make a pH=3 buffer solution, one possible choice from the given ingredients is 2.5M \(CH_3COOH\) (acetic acid) and its conjugate base, 2.0M KHCOO (potassium acetate). If only one component is available, it is not possible to create a solution that has both a weak acid and its conjugate base, which are necessary for a buffer.
A buffer solution consists of a weak acid and its conjugate base (or a weak base and its conjugate acid) that can resist changes in pH when small amounts of acid or base are added. In this case, the desired pH is 3, so we need an acidic buffer.
From the given ingredients, 2.5M \(CH_3COOH\) (acetic acid) is a weak acid, and its conjugate base is the acetate ion (\(CH_3COO-\). To create a pH=3 buffer, we would combine the acetic acid with its conjugate base, which is potassium acetate (KHCOO). Therefore, the correct choice for the buffer solution would be 2.5M \(CH_3COOH\) and 2.0M KHCOO.
If only one component is available (either a weak acid or its conjugate base), it is not possible to create a buffer solution. Both the weak acid and its conjugate base are essential for maintaining the buffer's pH.
Learn more about buffer solution here:
https://brainly.com/question/31428923
#SPJ11
A reaction vessel contains 50.0 grams of sodium metal and chlorine gas. How many grams of chlorine gas are needed to produce 75.0 grams of sodium chloride? If this amount of chlorine gas is added to the vessel, how much sodium metal would remain after the reaction goes to completion?
Answers
Answer:
- \(m_{Cl_2}=45.5gCl_2\)
- \(m_{Na}^{unreacted}=20.5g\)
Explanation:
Hello,
In this case, we consider the undergoing chemical reaction as:
\(2Na+Cl_2\rightarrow 2NaCl\)
Thus, for 75.0 grams of sodium chloride, the following grams of chlorine are required (consider their 2:1 molar ratio):
\(m_{Cl_2}=75.0gNaCl*\frac{1molNaCl}{58.44gNaCl}*\frac{1molCl_2}{2molNaCl}*\frac{70.9gCl_2}{1molCl_2} \\ \\m_{Cl_2}=45.5gCl_2\)
Then, for the unreacted grams of sodium, we first compute the actually reacted grams by considering the 2:2 molar ratio between sodium chloride and sodium:
\(m_{Na}=75.0gNaCl*\frac{1molNaCl}{58.44gNaCl}*\frac{2molNa}{2molNaCl}*\frac{23.0gNa}{1molNa} \\ \\m_{Na}=29.5gNa\)
Finally, we subtract:
\(m_{Na}^{unreacted}=50.0g-29.5g\\\\m_{Na}^{unreacted}=20.5g\)
Regards.
How does oxygen obey the octet rule
Answers
Answer:
oxygen obey the octet rule when reacting to form compounds by adding 2 electrons.
Explanation:
Answer:
See below.
Explanation:
An oxygen atom, in free state has 8 electrons, 2 in the first shell, and 6 in the second. It can only achieve stability if the valency of the valence shell is 0. In order for this, it combines with another atom with the same valency, such as magnesium or another oxygen atom to obey the octet rule. Hope it helps.
write the ionic equation for dissolution and the solubility product (ksp) expression for each of the following slightly soluble ionic compounds: (a) pbcl2 (b) ag2s (c) sr3(po4)2 (d) srso4
Answers
The ionic equation for dissolution and the solubility product (ksp) expression for each of the following slightly soluble ionic compounds.
(a) For PbCl2:
Dissolution: PbCl2 (s) → Pb²⁺ (aq) + 2Cl⁻ (aq)
Ionic equation: PbCl2 (s) ⇌ Pb²⁺ (aq) + 2Cl⁻ (aq)
Solubility product (Ksp) expression: Ksp = [Pb²⁺][Cl⁻]²
(b) For Ag2S:
Dissolution: Ag2S (s) → 2Ag⁺ (aq) + S²⁻ (aq)
Ionic equation: Ag2S (s) ⇌ 2Ag⁺ (aq) + S²⁻ (aq)
Solubility product (Ksp) expression: Ksp = [Ag⁺]²[S²⁻]
(c) For Sr3(PO4)2:
Dissolution: Sr3(PO4)2 (s) → 3Sr²⁺ (aq) + 2PO₄³⁻ (aq)
Ionic equation: Sr3(PO4)2 (s) ⇌ 3Sr²⁺ (aq) + 2PO₄³⁻ (aq)
Solubility product (Ksp) expression: Ksp = [Sr²⁺]³[PO₄³⁻]²
(d) For SrSO4:
Dissolution: SrSO4 (s) → Sr²⁺ (aq) + SO₄²⁻ (aq)
Ionic equation: SrSO4 (s) ⇌ Sr²⁺ (aq) + SO₄²⁻ (aq)
Solubility product (Ksp) expression: Ksp = [Sr²⁺][SO₄²⁻]
Learn more about solubility products (ksp) at brainly.com/question/17101393
#SPJ11
HELP ANYONE PLEASEE?? TY!!!

Answers
Answer:
so the order is grass, which gets eaten by the grasshopper, which is eaten by the frog, which gets eaten by the snake. grass wpuld be at top, snake at bottom.
Explanation:
hope this helps
In an ecosystem, the first trophic level is for grass followed by , grasshopper,snake and then frog.
What is an ecosystem?An ecosystem is defined as a system which consists of all living organisms and the physical components with which the living beings interact. The abiotic and biotic components are linked to each other through nutrient cycles and flow of energy.
Energy enters the system through the process of photosynthesis .Animals play an important role in transfer of energy as they feed on each other.As a result of this transfer of matter and energy takes place through the system .Living organisms also influence the quantity of biomass present.By decomposition of dead plants and animals by action of microbes nutrients are released back in to the soil.
Learn more about ecosystem,here:
https://brainly.com/question/13979184
#SPJ7
What type of particles move to create electricity
Again for the subject there was no sentence

Answers
Answer:
Electrons
Explanation:
Accurately measure approximately ( within 10%)1.00g of sodium hydroxide.
Answers
Using a digital weighing scale to accurately measure approximately ( within 10%)1.00g of sodium hydroxide.
Digital weighing has a precision of at least 0.1 g. The scale is calibrated and placed on a stable surface.
Place a weighing boat or a piece of weighing paper on the scale and press the tare button to reset the scale to zero.
Using a spatula, transfer some of the sodium hydroxide pellets or powder to the weighing boat or paper until the scale reads around 1.1 g.
Using the spatula, remove small amounts of sodium hydroxide at a time until the scale reads exactly 1.0 g.
Once you have reached 1.0 g, transfer the sodium hydroxide to a clean and dry container, such as a beaker or a test tube.
To learn more about the digital weighing, follow the link:
https://brainly.com/question/6551890
#SPJ1
Which of the following has a larger mass:
2 mole of water
2 mole of carbon dioxide
2 mole of ozone
Answers
Answer:
2 mole of water is greater than 2 mole of carbon dioxide which is greater than 2 mole of ozone
Explanation:
water has 36g
carbon dioxide has 44g
ozone 48g
Frank has a paper clip. It has a mass of 15g and a volume of 3cm. What is its density?
27 g/cm3
5 g/cm3
0.2 g/cm
18 g/cm
Answers
Answer:
\(\boxed {\tt 5 \ g/cm^3}\)
Explanation:
Density can be found by dividing the mass by the volume. Use the following formula.
\(d=\frac{m}{v}\)
The mass of the paper clip is 15 grams.
The volume of the paper clip is 3 cubic centimeters.
\(m= 15 \ g\\v= 3 \ cm^3\)
Substitute the values into the formula.
\(d=\frac{15 \ g}{3 \ cm^3}\)
Divide.
\(d= 5 \ g/cm^3\)
The density of the paper clip is 5 grams per cubic centimeters, or 5 g\cm³
What is [OH-1] for a 0.0050 M KOH solution?
(A) 2.5 x 10-5. (B) 0.0025. (C) 2.30. (D) 1 x 10-5. (E) 5.0 x 10-3.
Answers
The answer is (E) 5.0 x 10^-3. KOH is a strong base, meaning it dissociates completely in water to form OH- ions
At equilibrium, a solution of a weak base in water is a mixture of the nonionized base, the conjugate acid of the weak base, and hydroxide ion with the nonionized base present in the greatest concentration. Thus, a weak base increases the hydroxide ion concentration in an aqueous solution (but not as much as the same amount of a strong base). . The concentration of OH- ions in a 0.0050 M KOH solution can be calculated using the equation:
[OH-] = Kw / [H+]
where Kw is the ion product constant for water (1.0 x 10^-14 at 25°C).
Since KOH is a strong base, we can assume that [OH-] is equal to the concentration of KOH (0.0050 M). Therefore:
[OH-] = 0.0050 M
To know more about ionic equilibrium, click here:-
https://brainly.com/question/31217743
#SPJ11
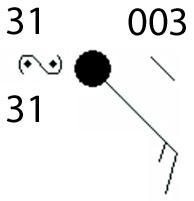
The picture below shows a weather station model at a location.
What type of weather is expected at the location?
A. fog
B. haze
C. dust storm
D. freezing rain
Answers
The type of weather expected according to the symbol is freezing rain. Option D
How can you identify the weather in the picture?The weather symbol for freezing rain is a capital S with one or two points.
The points represent the intensity of the freezing rain. One point indicates light freezing rain, while two points indicate moderate freezing rain.
Freezing rain is known as a type of precipitation that occurs when rain falls through a layer of cold air and freezes on contact with the ground or other surfaces.
It can cause dangerous driving conditions, power outages, and other disruptions.
Find more exercises on weather symbols;
https://brainly.com/question/29559036
#SPJ1
If the element gallium has an atomic number of 31 and an atomic mass of 70, how many neutrons does it have
Answers
Please mark brainliest I need 1 more!!!!!!!!!!
Help pleaseedeeeeeeeeeeeeeeee

Answers
Answer:
the answer to this question here is A
Explanation:
trust me
Answer:
Acid and Salt
Explanation:
Common hydrogen compounds
Hydrogen combines with other elements, forming a number of compounds, including common ones such as water (H2O), ammonia (NH3), methane (CH4), table sugar (C12H22O11), hydrogen peroxide (H2O2) and hydrochloric acid (HCl), according to Jefferson Lab.
The specific heat capacity of methane gas is 2.20 J/g°C. How many joules of heat are needed to raise the temperature of 5.00 g of methane from 22.0°C to 57.0°C?
a-79.5 J
b-385J
c-869 J
d-0.0126 J
Answers
Answer:
b - 385 j
Explanation:
Non-ferrous metal is NOT hardenable by heat treatment; it must
gain strength through a process such as tempering. Is this
statement TRUE or FALSE?
Group of answer choices
True
False
Answers
The statement is FALSE. Non-ferrous metals can be hardened by heat treatment, although the mechanisms and processes involved may differ from ferrous metals.
Heat treatment techniques such as precipitation hardening can be used to increase the strength of non-ferrous metals. Non-ferrous metals are metals or alloys that do not include iron (or iron allotropes, such as ferrite, etc.) in significant quantities. Non-ferrous metals are employed because they have desired qualities like reduced weight (for example, aluminium), greater conductivity (for example, copper), non-magnetic characteristics, or corrosion resistance (for example, zinc), even though they are often more expensive than ferrous metals. In the iron and steel sectors, several non-ferrous materials are also employed. Bauxite, for instance, is used as a flux in blast furnaces, whereas wolframite, pyrolusite, and chromite are utilised to create ferrous alloys.
To know more about Non-ferrous metals
https://brainly.com/question/33291477
#SPJ11
Avogadro's number is the number of particles in one gram of a carbon-12 atom. true false
Answers
False.
12 grams of carbon-12 constitutes 1 mole of carbon. 1 mole of any substance contains Avogadro number of particles.
A mole is a unit of measurement in chemistry.
one mole of something is equal to as many of that something as there are atoms in 12 grams of the isotope carbon-12.
Avogadro’s wide variety, the wide variety of gadgets in a single mole of any substance (described as its molecular weight in grams), is the same as 6.02214076 × 1023. The gadgets can be electrons, atoms, ions, or molecules, relying on the character of the substance and the individual of the reaction.
To learn more about carbon-12 atoms, please refer to the link below:
https://brainly.com/question/1548721
Answer: False
Explanation: Did the exam
Calculate the energy in joules of a wave particles with a wave length of 6.5×10^6 nm (infrared region)
Answers
Answer:
The energy of the wave particle is 3.058 x 10⁻²³ J.
Explanation:
Given;
wavelength of the wave particle, λ = 6.5 x 10⁶ nm = (6.5 x 10⁶) x 10⁻⁹ m
The energy of the wave particle is calculated as;
\(E = hf\\\\E = \frac{hc}{\lambda}\)
where;
h is Planck's constant = 6.626 x 10⁻³⁴ J/Hz
c is speed of light = 3 x 10⁸ m/s
\(E = \frac{(6.626 \times 10^{-34})(3\times 10^8)}{6.5 \times 10^6 \times 10^{-9}} \\\\E = 3.058 \times 10^{-23} \ J\)
Therefore, the energy of the wave particle is 3.058 x 10⁻²³ J.
Which particle model diagram represents xenon at stp?
Answers
The model that should show the corresct representation of xenon gas is one in which the gas molecules are isolated and monoatomic.
What is a noble gas?A noble gas is a member of group 18 of the periodic table. Noble gases are known not to interact with each other and occur as monoatomic particles.
The images are not shown here hence the question is incomplete. However, we do know that any of the models that show individual monoatomic particles is a representation of xenon gas.
Learn more about noble gas: https://brainly.com/question/2094768
In the reaction 2 H2 + O2 + 2 H20 what is the coefficient of oxygen?
Answers
Which coefficients correctly balance the formula equation below? NH 4 NO 2 (s) N 2 (g)+H 2 O(l) A. 1, 2, 2 C. B. 2, 1, 2 D. 1, 2, 1 1, 1, 2
Answers
The coefficients which correctly balance the formula for the equation of reaction of NH₄ and NO₂ is 1,1,1,2.
What is a Balanced Chemical reaction?This is the type of reaction in which the atoms of the elements of the reactants equal that of the product.
The balanced reaction is seen below where 2 was added to H₂O so as to balance the atoms of oxygen on both sides.
NH₄ + NO₂ (s) ⇒ N₂ (g)+2H₂O(l)
Read more about Balanced Chemical reaction here https://brainly.com/question/15355912
how does the composition and management of solid waste vary
around the world
Answers
The composition and management of solid waste can vary significantly based on several factors such as geographical location, population size, level of industrialization, cultural practices, and available infrastructure.
Here are some ways in which the composition and management of solid waste can vary:
1. Composition: The types of waste generated can vary depending on the activities and industries in the area. In urban areas, the waste stream typically consists of household waste, commercial waste, construction and demolition waste, and institutional waste. In industrial areas, there may be a higher proportion of industrial and hazardous waste. Rural areas may have more agricultural and organic waste.
2. Quantity: The amount of solid waste generated can vary depending on the population density, consumption patterns, and economic activities in the area. Urban areas tend to generate larger quantities of waste compared to rural areas due to higher population density and commercial activities.
3. Recycling and Waste Diversion: The extent of recycling and waste diversion practices can vary. Some regions have well-established recycling programs and waste segregation practices, leading to higher rates of waste diversion from landfills. In contrast, other areas may have limited recycling infrastructure, resulting in lower recycling rates.
4. Disposal Methods: The methods used for waste disposal can vary. Common methods include landfilling, incineration, composting, and anaerobic digestion. Developed countries often have advanced waste management systems that incorporate multiple disposal methods, whereas developing countries may rely more on traditional landfilling practices.
5. Legal and Regulatory Framework: The regulations and policies governing solid waste management can differ between regions and countries. Some areas have stringent waste management regulations in place, ensuring proper waste handling, disposal, and environmental protection. In contrast, other regions may have less comprehensive regulations, leading to inadequate waste management practices.
6. Infrastructure and Resources: The availability of infrastructure and resources for waste management can vary. Developed regions generally have better waste management infrastructure, including waste collection systems, recycling facilities, and treatment plants. In contrast, developing regions may face challenges in terms of limited resources, inadequate infrastructure, and financial constraints.
It is important to note that effective solid waste management requires a holistic approach that encompasses waste reduction, recycling, proper disposal, and awareness among individuals and communities. Local governments and authorities play a crucial role in implementing sustainable waste management practices based on the specific needs and circumstances of their respective regions.
To know more about solid waste
https://brainly.com/question/30758552
#SPJ11
Kw=[H3O+][OH-]=1.0x10^-14 At 25*c
Based on the information above, which of the following is true for a sample of pure water at 25°C?
A: [H₂0+] = 7.0 M
B: [OH-] = 1.0 x 10-¹4 M
C:pH = 10-7
D: POH = 7.00
Answers
A sample of pure water at 25°C has a pH of 7.00.
we can use the Kw expression to calculate the concentration of either ion:
Kw = [H3O+][OH-]
Since pure water is neutral, [H3O+] = [OH-]. Therefore:
[H3O+] = [OH-] = sqrt(Kw) = sqrt(1.0 x 10^-14) = 1.0 x 10^-7 M
So, the correct answer is C: pH = 7.00.
The pH of a solution is defined as the negative logarithm of the H3O+ concentration:
pH = -log[H3O+]
Substituting the value we found for [H3O+], we get:
pH = -log(1.0 x 10^-7) = 7.00
Therefore, a sample of pure water at 25°C has a pH of 7.00.
learn more about Kw here
https://brainly.com/question/30901531
#SPJ9