Community funding may suffer when a company receive a _________ which may mean less money to pay for public service
Answers
Answer:
Public substance abuse treatment programs have traditionally relied on three funding Such an agency may already have funding to provide services to individuals However, no such restrictions apply to payments received through manage.
Related Questions
If 220 grams of C3H8 react with 20 grams of O2, how much CO2 can be formed?
Answers
Answer:
Explanation:
The bonding within the molecules or compounds directly affects its solubility. The_______ bonds within hexane, CH14. prevent the compound from being dissolved by a solvent like water , H₂O.A) shared ionicB) polar covalentC) nonpolar covalentD)Electrostatic metallic
Answers
Water is a polar substance. Polar solvents dissolve polar substances. Now, the bond between carbon and hydrogen is a covalent bond, because the electronegativity difference between these two elements is less than 1.7.
Therefore, the low solubility of hexane is due to the fact that hexane is nonpolar with covalent bonds.
Answer: C) Nonpolar covalent
Element Y has two naturally occurring isotopes. The most dominant isotope has a mass of 114.3789 amu and a percent abundance of 64.23%. What is
the mass of the second isoptope if the average atomic mass is 128.4359 amu? Remember that the two percentages will have to sum to 100%.
Answer to the correct number of sigtigs including units in your answer.
(to receive full credit, work must be submitted for this question)
Answers
Answer:
153.6771 amu
Explanation:
From the question given above, the following data were:
Isotope A:
Mass of A = 114.3789 amu
Abundance (A%) = 64.23%
Isotope B:
Mass of B =.?
Abundance (B%) = 100 – A%
Abundance (B%) = 100 – 64.23
Abundance (B%) = 35.77%
average atomic mass of Element Y = 128.4359 amu
The mass of the 2nd isotope (i.e isotope B) can be obtained as follow:
Average atomic mass = [(Mass of A × A%)/100] + [(Mass of B × B%)/100]
128.4359 = [(114.3789 × 64.23)/100] + [(Mass of B × 35.77) /100]
128.4359 = 73.4656 + (Mass of B × 0.3577)
Collect like terms
128.4359 – 73.4656 = Mass of B × 0.3577
54.9703 = Mass of B × 0.3577
Divide both side by 0.3577
Mass of B = 54.9703 / 0.3577
Mass of B = 153.6771 amu
Therefore, the mass of the 2nd isotope is 153.6771 amu
please answer this as quickly as possible

Answers
Explanation:
I. Because an explosion is a very fast exothermic reaction
ll. it depends on the it's particle size
. One of the essential minerals in the human body is salt. How much salt (NaCl) is in the average adult human body?
Answers
Answer:
200g or 40 teaspoons
Explanation:
An average human, weighing about 50 pounds, has about 200 g or 40 tps of NACl
how to tell if a functional group is acidic or basic
Answers
Determining whether a functional group is acidic or basic depends on its ability to either donate or accept a proton (H+). Here are some general guidelines to help you assess the acidity or basicity of a functional group:
1. Acidity:
a. Look for functional groups that have an acidic hydrogen directly bonded to an electronegative atom, such as oxygen or a halogen. Examples include carboxylic acids (–COOH) and phenols (–OH on an aromatic ring).
b. Consider the stability of the resulting conjugate base. If the conjugate base is stabilized through resonance or delocalization of the negative charge, the functional group is more acidic. For example, the carboxylate ion (–COO-) is stabilized through resonance.
2. Basicity:
a. Look for functional groups that contain lone pairs of electrons, which can readily accept a proton. Common examples include amines (–NH2) and amides (–CONH2).
b. Consider the availability of lone pairs. The more accessible the lone pairs are, the more basic the functional group. For example, primary amines have more available lone pairs than tertiary amines and are, therefore, more basic.
It's important to note that the acidity or basicity of a functional group can also be influenced by its environment, neighboring groups, and other factors. These guidelines provide a general starting point, but there may be exceptions and variations based on specific compounds and circumstances.
To know more about functional group refer here
https://brainly.com/question/31332495#
#SPJ11
What is the Aluminum chlorite formula
Answers
Answer:
Explanation:
Aluminum chlorite formula is :Al(ClO2)3
Why is it important for the kitchen manager to make sure food is fresh?
Answers
The rate of hormone removal is called the __________, and the length of time required to clear 50% of the hormone from the blood is the __________.
Answers
Answer:
1st answer clearance of hormones
the 2nd question im not sure but it take 4 weeks
Explanation:
32.According to the solubility graph, at 30°C, which of the following compounds is the most soluble in 100 grams of water?Select one:a. NaClb. Yb2(SO4)3c. KNO3d. NaNO3

Answers
According to the graph presented, we can see that NaNO3 has a higher solubility at 30°C, as we can see more than 100 grams of NaNO3 can be dissolved in 100 grams of water, whereas all other solutes will have a way lower value. Letter D
Order the steps in the scientific method.
Plan an investigation
Interpret data
Form hypothesis
Draw a conclusion
Answers
The ordered steps of the scientific method include forming a hypothesis, planning an investigation, interpreting data, and drawing a conclusion.
What is the scientific method?The scientific method is a series of sequential steps to obtain scientific evidence. i.e., evidence from a given aspect of the real world, which is used to draw final conclusions.
This empirical evidence is characterized to be testable, which means we can obtain the same result by applying the same experimental and/or observation procedures.
In conclusion, the ordered steps of the scientific method include forming a hypothesis, planning an investigation, interpreting data, and drawing a conclusion.
Learn more about the scientific method here:
https://brainly.com/question/17216882
#SPJ1
Answer:
1. Form hypothesis
2. Plan an investigation
3. Interpret data
4. Draw a conclusion
Explanation:
hope this helps :)
Each step in any energy conversion process will _____.
create energy
gain energy
lose energy
destroy energy
Answers
the correct answer is dissapate...but it is
not here so i think relativly the answer is destroy
Help with theses two different problems!
1.) 125mL of what is added to 45.3mL of 0.71m NaOH solution
2.) 550mL of water is added to 125mL of 3.01M KOH solution
Answers
1. the final concentration of NaOH after adding 125 mL of water to 45.3 mL of 0.71 M NaOH solution is approximately 0.189 M.
2. the final concentration of KOH after adding 550 mL of water to 125 mL of 3.01 M KOH solution is approximately 0.557 M.
1.) If 125 mL of water is added to 45.3 mL of a 0.71 M NaOH solution, the resulting solution will be a diluted NaOH solution. The addition of water will increase the total volume while reducing the concentration of NaOH. To determine the final concentration of NaOH, we need to consider the conservation of moles.
First, let's calculate the moles of NaOH in the initial solution:
moles of NaOH = volume (in L) × concentration (in M)
moles of NaOH = 0.0453 L × 0.71 M = 0.0321433 moles
After adding 125 mL (0.125 L) of water, the total volume of the solution becomes 0.0453 L + 0.125 L = 0.1703 L.
To find the final concentration, we divide the moles of NaOH by the total volume:
final concentration of NaOH = moles of NaOH / total volume
final concentration of NaOH = 0.0321433 moles / 0.1703 L ≈ 0.189 M
Therefore, the final concentration of NaOH after adding 125 mL of water to 45.3 mL of 0.71 M NaOH solution is approximately 0.189 M.
2.) If 550 mL of water is added to 125 mL of a 3.01 M KOH solution, the resulting solution will also be a diluted solution. Again, we will apply the conservation of moles to determine the final concentration of KOH.
First, calculate the moles of KOH in the initial solution:
moles of KOH = volume (in L) × concentration (in M)
moles of KOH = 0.125 L × 3.01 M = 0.37625 moles
After adding 550 mL (0.55 L) of water, the total volume of the solution becomes 0.125 L + 0.55 L = 0.675 L.
To find the final concentration, divide the moles of KOH by the total volume:
final concentration of KOH = moles of KOH / total volume
final concentration of KOH = 0.37625 moles / 0.675 L ≈ 0.557 M
For more such questions on concentration visit:
https://brainly.com/question/28564792
#SPJ8
help help help plsss
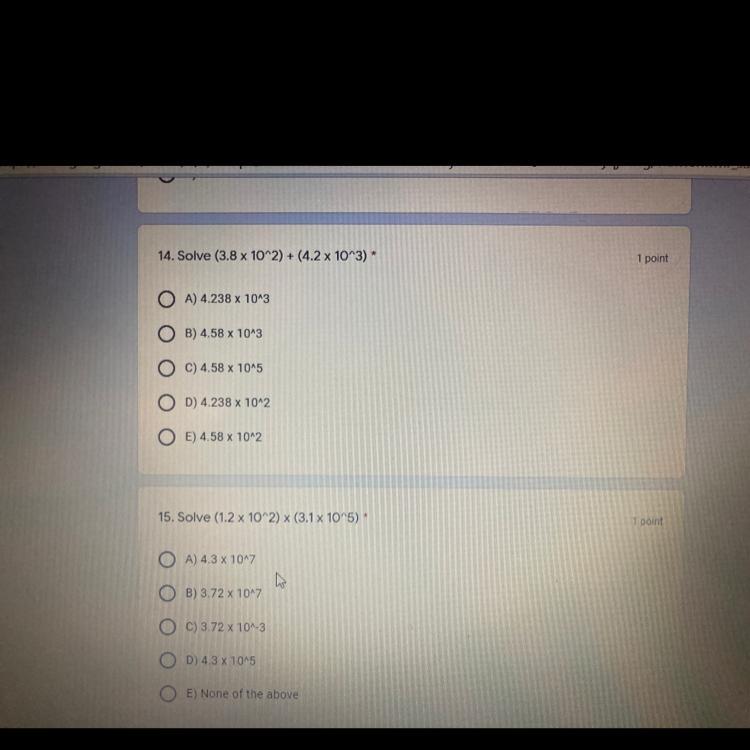
Answers
Answer:
4.58×10^3
3.72×10^7
Explanation:
3.8×10^2 =380
+ 4.2×10^3=4200
= 4580 = 4.58×10^3
Using Lewis structures and formal charge, which of the following ions is most stable? The atoms bonded in the order shown. OCN^- ONC^- NOC^- OCN^1 ONC^- NOC^- None of these ions are stable according to Lewis theory. All of these compounds are equally stable according to Lewis theory.
Answers
All three ions have a formal charge of 0, they are all stable according to Lewis theory. However, we can further analyze their stability based on their resonance structures and bond energies, which is beyond the scope of this question. Therefore, the answer is e) All of these compounds are equally stable according to Lewis theory .
To determine which of the ions is most stable using Lewis structures and formal charge, we can infer the following from lewis structures of each ion :
a) OCN^-
O has 6 valence electrons, C has 4, and N has 5. To have a complete octet, O shares two electrons with C and one electron with N. The N atom has a lone pair of electrons, and the negative charge is on the O atom.
b) ONC^-
O has 6 valence electrons, N has 5, and C has 4. To have a complete octet, O shares two electrons with N and one electron with C. The C atom has a lone pair of electrons, and the negative charge is on the O atom.
c) NOC^-
N has 5 valence electrons, O has 6, and C has 4. To have a complete octet, N shares two electrons with O and one electron with C. The C atom has a lone pair of electrons, and the negative charge is on the N atom.
To determine the formal charge of each atom, we use the formula:
formal charge = valence electrons - non-bonded electrons - half of bonded electrons
a) O has 6 valence electrons, is bonded to two other atoms (C and N), and has 4 non-bonded electrons. Formal charge = 6 - 4 - 2 = 0. C has 4 valence electrons, is bonded to two other atoms (O and N), and has 2 non-bonded electrons. Formal charge = 4 - 2 - 4/2 = 0. N has 5 valence electrons, is bonded to two other atoms (O and C), and has 3 non-bonded electrons. Formal charge = 5 - 3 - 4/2 = 0.
b) O has 6 valence electrons, is bonded to two other atoms (N and C), and has 4 non-bonded electrons. Formal charge = 6 - 4 - 2 = 0. N has 5 valence electrons, is bonded to two other atoms (O and C), and has 3 non-bonded electrons. Formal charge = 5 - 3 - 4/2 = 0. C has 4 valence electrons, is bonded to two other atoms (O and N), and has 2 non-bonded electrons. Formal charge = 4 - 2 - 4/2 = 0.
c) N has 5 valence electrons, is bonded to two other atoms (O and C), and has 3 non-bonded electrons. Formal charge = 5 - 3 - 4/2 = 0. O has 6 valence electrons, is bonded to two other atoms (N and C), and has 4 non-bonded electrons. Formal charge = 6 - 4 - 2 = 0. C has 4 valence electrons, is bonded to two other atoms (N and O), and has 2 non-bonded electrons. Formal charge = 4 - 2 - 4/2 = 0.
Since all three ions have a formal charge of 0, they are all stable according to Lewis theory. However, we can further analyze their stability based on their resonance structures and bond energies, which is beyond the scope of this question. Therefore, the answer is e) All of these compounds are equally stable according to Lewis theory , i.e, all the given ions are equally stable ions.
Learn more about formal charge : https://brainly.com/question/28446255
#SPJ11
Answer:
OCN^-
Explanation:
Out of all of the answers, OCN^- is the most stable ion.
Nate and Clara are drawing pictures with markers. There are 8 markers in a set. Nate has 9 markers and Clara has 7. What can Nate and Clara do so that each of them has a full set?
Answers
9-1=8
7+1=8
what is the type of weak bond between the hydrogen of one molecule and the nitrogen of another molecule, where the two don't actively share an electron? group of answer choices hydrogen bond ionic bond disulfide bond hydrophobic bond covalent bond
Answers
The weak bond between the hydrogen atom of one molecule and the nitrogen atom of another molecule, where the two don't actively share an electron, is called hydrogen bond. Option A is correct.
A hydrogen bond is a type of intermolecular attraction that occurs when a hydrogen atom, already covalently bonded to one electronegative atom, interacts with another electronegative atom.
In this case, the hydrogen is bonded to a highly electronegative nitrogen atom, creating a partially positive charge on the hydrogen and a partially negative charge on the nitrogen, allowing for electrostatic attraction between the two molecules.
Hydrogen bonds are relatively weak compared to covalent bonds, but they play a critical role in many biological and chemical processes. For example, hydrogen bonds help hold together the two strands of DNA, which is critical for the proper functioning of genetic information.
Hence, A. is the correct option.
To know more about hydrogen bond here
https://brainly.com/question/10904296
#SPJ4
--The given question is incomplete, the complete question is
"What is the type of weak bond between the hydrogen of one molecule and the nitrogen of another molecule, where the two don't actively share an electron? group of answer choices A) hydrogen bond B) ionic bond C) disulfide bond D) hydrophobic bond E) covalent bond."--
Aqueous magnesium chloride is added to aqueous silver nitrate. A white precipitate forms. Write the chemical equation for this reaction.
Answers
Answer:
1. Molecular equation
BaCl2(aq) + 2AgNO3(aq) –> 2AgCl(s) + Ba(NO3)2 (aq)
2. Complete Ionic equation
Ba²⁺(aq) + 2Cl¯(aq) + 2Ag⁺(aq) + 2NO3¯ (aq) —> 2AgCl(s) + Ba²⁺(aq) + 2NO3¯(aq)
3. Net ionic equation
Cl¯(aq) + Ag⁺(aq) —> AgCl(s)
Explanation:
Answer:
The symbol for magnesium chloride is MgCl2 and silver nitrate is Ag(NO3)2 so the equation will be :
MgCl2 + Ag(NO3)2 → AgCl2 + Mg(NO3)2
What element is in period 6 with 2 valence electrons?
Answers
Answer:
Barium
Explanation:
I think that's the answer
The element in period 6 with 2 valence electrons is Barium.
The periodic table has been organized into periods and groups. The period is in an horizontal arrangement while group is vertical arrangement. The elements that can be found in period 6 are Beryllium, Magnesium, Calcium, Strontium, Barium etc. Elements in the same group have the same number of valence electrons. This means that elements in group 2 have 2 valence electrons. Barium possess two valence electrons because it is in group 2 and it is also in period 6. Therefore, the element in period 6 with 2 valence electrons is Barium.Learn more at: https://brainly.com/question/24089206?referrer=searchResults
Consider the diffusion of oxygen through a low-density polyethylene (LDPE) sheet 15 mm thick. The pressures of oxygen at the two faces are 2000 kPa and 150 kPa, which are maintained constant. Assuming conditions of steady state, what is the diffusion flux [in [(cm3 STP)/cm2-s] at 298 K
Answers
The diffusion flux of oxygen through the LDPE sheet can be calculated using Fick's first law of diffusion:
J = -D*(delta C/delta x)
where J is the diffusion flux, D is the diffusion coefficient of oxygen in LDPE, delta C is the difference in oxygen concentration across the sheet, and delta x is the thickness of the sheet.
Assuming ideal gas behavior, we can use the following expression to convert between partial pressure and concentration:
C = (P/(R*T))
where C is the concentration in mol/cm^3, P is the partial pressure in Pa, R is the gas constant (8.314 J/mol-K), and T is the temperature in Kelvin.
Using the given pressures and the ideal gas law, we can calculate the concentration difference across the sheet as follows:
delta C = (P1/(RT)) - (P2/(RT))
delta C = ((2000 kPa)*1000 Pa/kPa)/(8.314 J/mol-K * 298 K) - ((150 kPa)*1000 Pa/kPa)/(8.314 J/mol-K * 298 K)
delta C = 0.0412 mol/cm^3
The diffusion coefficient of oxygen in LDPE at 298 K is approximately 1.4x10^-9 cm^2/s.
Plugging in the given values, we get:
J = -D*(delta C/delta x)
To know more about coefficient visit :
https://brainly.com/question/28975079
#SPJ11
After obtaining vital signs, which prescribed medication should the nurse hold when caring for a client on the cardiac unit?ExhibitYou answered this question Correctly1. Rosuvastatin2. Enalapril3. Digoxin4. Clopidogrel T - 98 ° (36.7°)P - 74R - 20BP - 88/50
Answers
Based on the vital signs provided, the nurse should hold Digoxin when caring for the client on the cardiac unit.
Digoxin is a medication commonly prescribed to treat heart conditions, such as atrial fibrillation and heart failure. However, it can cause adverse effects on the heart and other organs if the dose is not carefully monitored, especially if the client's vital signs are not within a certain range. In this case, the client's blood pressure is lower than the normal range, which can indicate hypotension. Digoxin can further lower blood pressure and cause adverse cardiac effects in clients with low blood pressure. Therefore, the nurse should hold the medication and contact the healthcare provider to adjust the dosage or withhold the medication until the client's blood pressure stabilizes.
It is crucial for nurses to monitor their client's vital signs before administering medications, especially for clients on the cardiac unit. Vital signs, such as blood pressure, heart rate, and temperature, provide valuable information about the client's health status and can help identify potential adverse effects of medication. In this scenario, the nurse correctly identified that the client's low blood pressure warrants holding Digoxin. By doing so, the nurse can prevent adverse events and promote the client's safety and well-being.
To learn more about blood pressure click here : brainly.com/question/14572872
#SPJ11
complete the paragraph to describe binary systems
Answers
A binary star system is made of stars, two of which is brighter than the other.
Astronomers are able to detect the dimmer star because its gravity causes the bright star to wobble
Astronomers can also spot the dimmer star by observing a phenomenon called a(n) eclipsing binary. This happens when the dim star passes in front of the bright star.
When one astronomical body is obscured by another or when a body moves between it and the observer, this is known as an eclipse.Partial solar eclipses can be seen if you are in the moon's penumbra during a solar eclipse. Solar eclipses happen when the sun is partially blocked by the moon.When the sun, moon, and earth align, the earth covers the moon's view of the sun, causing lunar eclipses.To know more about eclipses visit : https://brainly.com/question/12075389
#SPJ9
Question:
In a laboratory demonstration, a balloon filled with methane and oxygen was exposed to a
flame. The result was a brief, large flame. The students were asked to formulate an equation for
the reaction. One answer is below.
CH, + 0 = CO,
This equation is incorrect.
A. Explain how and why it is incorrect
B. What would the correct equation be, and how do you know that?

Answers
Answer:
The laboratory demonstration consists of the following;
The compounds present in the combustion reaction = Methane, CH₄ and Oxygen, O₂
The chemical equation for the combustion reaction is given as follows;
CH₄ + 2O₂ → CO₂ + 2H₂O
Therefore;
A. The equation given as CH₄ + O → CO₂ is not correct because;
1) Oxygen gas exist as diatomic molecules, O₂, and given that the experiment involves the mixture of gases, the oxygen gas present which can exist as a separate compound, should be represented as O₂
2) The number of oxygen molecules in the reaction is two rather than one
3) The product also includes two molecules of water (vapor) H₂O
B. The correct equation for the reaction should be given as follows;
CH₄ + 2O₂ → CO₂ + 2H₂O
B i) The constituents of the equation is obtained by the knowledge of the fact that the combustion reaction of an organic substance such as methane in the presence of oxygen yields, carbon dioxide and water (vapor)
The equation showing the relative amounts the reacting compounds is by balancing the basic equation of the combustion of methane in the presence of oxygen
Explanation:
Barium sulfide decomposes into its
elements when heat and electricity are
applied.
Which reaction shows the balanced
equation for the decomposition?
A. 8BaS→ 8Ba + Sg
B. Ba₂S → 2Ba + S
C. BaS2
Ba + S₂
->
D. 2Ba₂S 4Ba + 2S
->
MARINA VINNSAMAN

Answers
A balanced equation obeys the law of conservation of mass. Here the balanced equation for the decomposition of Barium sulfide is 8 BaS → 8Ba + S₈. The correct option is A.
What is balanced equation?A chemical equation in which the amount of reactants and products on both sides of the equation are equal is defined as the balanced equation. The number of atoms of each element of the reactants and products are same on either side of the equation.
Here the balanced equation for the decomposition of Barium sulfide is denoted as:
8BaS→ 8Ba + S₈
The number of 'Ba' and 'S' atoms on both sides of the equation are equal.
Thus the correct option is A.
To know more about balanced equation, visit;
https://brainly.com/question/29769009
#SPJ1
Alice adds 0.17 g of magnesium nitrate to an empty graduated cylinder. What is the molar concentration after she adds enough water to bring the volume up to 30.0 mL?
Answers
The molar concentration of magnesium nitrate in the solution is 0.0350 M.
We can start by calculating the number of moles of magnesium nitrate:
moles of Mg(NO₃)₂ = mass / molar mass
= 0.17 g / (24.305 g/mol + 2x14.007 g/mol + 6x16.00 g/mol)
= 0.00105 mol
Next, we can calculate the molarity (M) of the solution using the formula:
M = moles of solute / volume of solution (in liters)
First, we need to convert the volume from milliliters (mL) to liters (L):
30.0 mL = 0.0300 L
Now we can plug in the values:
M = 0.00105 mol / 0.0300 L
= 0.0350 M
To know more about magnesium nitrate, here
brainly.com/question/28519426
#SPJ1
How many cm are in 7 km?
Answers
Answer:
70000 cm in 7km
Explanation:
There are 1000 m and 100 cm in 1 m in 1 km, so to find how many cm are in 7 km you need to do 10000 x 7. You get 70000
Have a nice day!
Answer:
700000cm
Explanation:
1 km consists of 1000m and 1 m consists of 100 cm so 7×1000×100=700000cm
An elemental gas has a mass of 10.3 g. If the volume is 58.4 L and the pressure is 758 Torr at a temperature of 2.5oC, what is the gas
Answers
Based on this molar mass, we can conclude that the gas is helium (He), which has a molar mass of approximately 4.0 g/mol. Therefore, the elemental gas in question is helium.
The ideal gas law states that
PV = nRT.
P represents pressure, V represents volume, n represents the amount of substance in moles, R represents the gas constant, and T represents temperature.
The gas constant varies depending on the units used for the other variables in the equation. The molar mass of the gas can be calculated from its density using the formula:
M = dRT/P,
where M is the molar mass, d is the density, R is the gas constant, and P and T are the pressure and temperature, respectively.
In this problem, we are given the mass, volume, pressure, and temperature of an elemental gas and asked to determine its identity. Since the mass of the gas is known, we can use the ideal gas law to determine the number of moles of gas present:
PV = nRT, or
n = PV/RT.
First, let's convert the temperature to Kelvin:
2.5oC + 273.15 = 275.65 K
Next, let's convert the pressure to atmospheres (atm) since the gas constant is typically given in those units: 758 Torr/760 Torr/atm = 0.997 atm
Now we can use the ideal gas law to solve for n:
n = PV/RT = (0.997 atm)(58.4 L)/(0.08206 L atm/K mol)(275.65 K)
n = 2.26 mol
Finally, we can use the mass and number of moles to calculate the molar mass of the gas:
M = m/n = 10.3 g/2.26 mol = 4.56 g/mol
to know more about gas elements visit:
https://brainly.com/question/5144239
#SPJ11
After the removal of carbon, the oxygen in co2 ends up.
a. true
b. false
Answers
The given statement, After the removal of carbon, the oxygen in CO₂ ends up is False.
Carbon dioxide (CO₂) is a compound made up of two elements, carbon and oxygen, in a ratio of one carbon atom for every two oxygen atoms. When carbon dioxide is removed, the oxygen atoms do not remain isolated - instead, they bond with other oxygen atoms from the surrounding environment, forming oxygen gas (O₂).
Oxygen gas is highly reactive and forms strong bonds with other oxygen atoms to form molecules of the natural gas O₂. The result is that the oxygen that was part of the carbon dioxide is no longer present - it has become part of the newly formed oxygen gas molecules.
Oxygen gas is present in the atmosphere and it is highly reactive and mobile, meaning that it can quickly move and form bonds with other elements. When carbon dioxide is removed, the oxygen atoms that were part of the molecule become part of oxygen gas instead, creating molecules of the natural gas O₂.
know more about environment here
https://brainly.com/question/5511643#
#SPJ11
An analyst was preparing standard solutions. While transferring the solid analyte to the volumetric flask, some amount of the solid stuck to the weigh paper and was not transferred. The measured concentrations came out a bit low for this solution. This is an example of:
Answers
The phenomenon described in the given question, where some amount of the solid stuck to the weigh paper and was not transferred, is an example of a volumetric error in the preparation of standard solutions.
A standard solution is a solution with a precisely known concentration of an element or a substance. They are prepared to a specific concentration by dissolving a known quantity of a pure substance in a particular solvent in a volumetric flask.
Volumetric errors are a type of systematic error that occurs when preparing standard solutions. They occur due to an error in the volume of solution added or when some of the solute gets lost during the transfer process. As a result, the measured concentration comes out a bit low for this solution.
To avoid such errors, it is essential to pay close attention to the precise amount of the analyte used and be careful while transferring it to the volumetric flask. Any loss of the solid during the transfer process will result in an incorrect concentration of the solution and can lead to incorrect results.
Thus, the correct answers is volumetric error.
To learn more about concentration :
https://brainly.com/question/17206790
#SPJ11
write two uses of echoes
Answers
Whales often use echoes in the form of echolocation to find their mates, which is a series of repeated sounds that are heard up to a certain radius in distance from the whale that produced the sound.
Sound reflection is used in the form of echoes so that only the users of the echoes can hear the sound. An example would be band players at practice, and how brick walls are used to surround the practice room so only they can hear what is being echoed.
Echo: When a sound wave is created in the air, some of it is reflected back to the listener while some of it is absorbed after hitting a reflector. Echo is the name for this reflected sound.
Utilizations for Echo:
1. Bats use the echolocation method to find their way because they are unable to see with their eyes. Bats can determine whether an object is in front of a sound by listening to how it is reflected. It uses this method when hunting its prey.
2. This method is also employed to determine undersea distance and ocean depth.
3. Estimating the separation between hills and mountains is also helpful.
Know more about echo at:
https://brainly.com/question/19579065