clerice midter
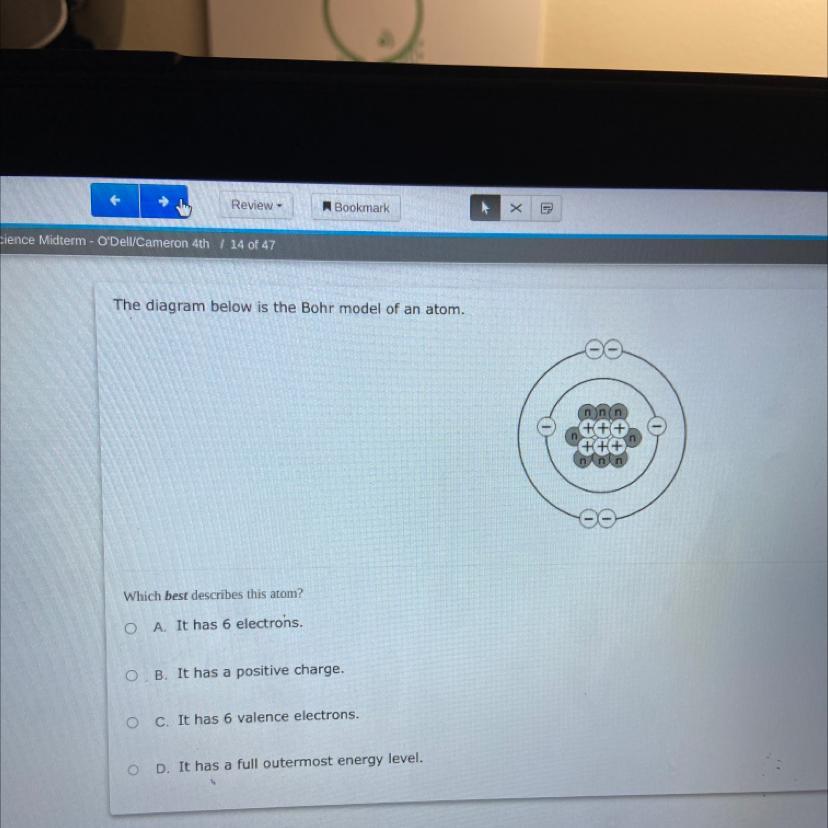
The diagram below is the Bohr model of an atom.
Which best describes this atom?
OA. It has 6 electrons.
OB. It has a positive charge.
O c. It has 6 valence electrons.
OD.
has a full outermost energy level.
Answers
The correct option is (A) - This Bohr Model of atom describes that there are a total of 6 electrons in the given figure.
What is Bohr Model of atom?The electrons are positioned in circular orbitals at particular distances from the central nucleus in the Bohr model of the atom. These orbits create electron shells or energy levels, which allow us to see how many electrons are present in each shell. The number and the letter "n" are used to identify these energy levels. The first energy level nearest to the nucleus, for instance, is represented by the 1n shell. Normally, an electron resides in the shell with the lowest energy, which is the one closest to the nucleus. A photon of light's energy can raise it to a higher energy shell, but this is an unstable position, and the electron quickly returns to the ground state.
Learn more about atom here:
https://brainly.com/question/30898688
#SPJ1
Related Questions
Identify the two gases jn the unknown mixtures.

Answers
Answer:
I believe Gases A and D
Explanation:
The lines in both gases match up with the lines in the unknown mixture.
Answer:
Fa (rt) and Nitrogen
Explanation:
Glycolysis depends on a continuous supply of: a. NADP b. pyruvate c. NAD+ d. NADH e. H2O
Answers
Glycolysis depends on a continuous supply of:
c. NAD+ (nicotinamide adenine dinucleotide)
During the process of glycolysis, glucose is broken down into pyruvate molecules. In this pathway, NAD+ is converted into its reduced form, NADH, by accepting electrons and hydrogen ions (H+) from certain steps of glycolysis. NADH acts as an electron carrier and plays a crucial role in the subsequent energy-yielding steps of cellular respiration.
The conversion of NAD+ to NADH in glycolysis is an essential step as it helps to maintain the balance of electron and energy flow in the pathway. NADH molecules produced in glycolysis are later used in the electron transport chain to generate ATP during oxidative phosphorylation. In this process, NADH donates its electrons to the respiratory chain, ultimately leading to the production of ATP.
Therefore, a continuous supply of NAD+ is necessary for the glycolytic pathway to continue functioning efficiently, ensuring the production of energy in the form of ATP.
Learn more about glycolysis here, https://brainly.com/question/1966268
#SPJ11
rank the species (carbonate chloride iodate and sulfate) from most to least soluble
Answers
The order of solubility from most to least can be given as carbonate>sulfate>iodate>chloride. Carbonates are the most soluble.
What is solubility?The maximum amount of a material that may dissolve in another is known as its solubility. A saturated solution is created when a solvent can dissolve its maximum quantity of solute before reaching equilibrium. A supersaturated solution results when extra solutes are dissolved past its equilibrium solubility point under specific circumstances.
Dissolution is the action of disintegrating. In contrast to the speed of solution, which specifies how rapidly a molecule dissolves in a solvent, solubility is not a feature of matter. The order of solubility from most to least can be given as carbonate>sulfate>iodate>chloride.
Therefore, the order of solubility from most to least can be given as carbonate>sulfate>iodate>chloride.
To know more about solubility, here:
https://brainly.com/question/14366471
#SPJ1
Help please. Will give brailiest

Answers
Answer:
i dont have a answer im just answering so you can give the person who answered brailiest :)
Explanation:
Cab someone give me a balanced chemical for my exam! <3

Answers
Answer: 4 NH₃ + 5 O₂ → 4NO + 6 H₂O
Explanation:
If we write out the chemical symbols for the molecules expressed in the question, along with the moles we get the following equation:
4 NH₃ + 5 O₂ → 4 NO + 6 H₂O
To know if the equation is balanced we can compare the Reantact side to the Product side for each element. If the equation is balanced, they would be equal.
Reactant Product
N 4 4
H 12 12
O 10 6+4 = 10
∴ the equation is balanced.
Protease enzymes break down proteins into amino acids. The breakdown
can be followed using albumen solution. Albumen is a protein which can
be mixed with water to make a solution. Albumen solution is cloudy.
When the protein is broken down, the solution goes clear.
Describe how you would carry out an experiment to test the effect of pH
on protease..
Include details of control variables and the range of pH values that you
would use?
Answers
It is found that a pH of 8 and a temperature of 37°C were most effective for the protease's maximum caseinolytic activity.
With plant leaf extracts at various stages of purification (crude soup is the initial supernatant after homogenization and centrifugation, 40% ammonium soup is the phosphate-dissolved pellet after 40% ammonium sulphate fractionation, and pooled soup is the final collection of pure fractions came from DEAE cellulose column), we have tested the protease activity in terms of caseinolytic activity. Prior to the protease experiment, all three samples were dialyzed to remove EDTA and protease inhibitor. Pure protein's protease activity was tested at various pH values (4–9) and temperatures (4–70°C). Different amounts of -casein were used as substrate for the enzyme activity assay for protease, which was carried out at pH-8 in 37°C according to the ideal conditions established by the preceding studies (ideal temperature and pH).
Here, the enzyme concentration was held constant while the substrate concentration (-casein) changed in the range (0.81, 1.6, 2.4, 4.03, 5.2) mg/ml.
To know more about protease activity, please refer:
https://brainly.com/question/28369482
#SPJ1
Which one is wrong I did them but it’s saying I got partial credit

Answers
A yellow-gold sphere has a mass of 15.2g when passed in the orange liquid the volume was 43ml
Answers
A yellow-gold sphere has a mass of 15.2 g when passes in the orange liquid with volume 43 mL
Density= mass/volume
Density = 15.2 g/ 43mL
Density = 0.35 g/mL
Question is to find density
How much excess reactant is left over when 17.0 g of potassium hydroxide (KOH) reacts with 20.0 g of iron (III) nitrate (Fe(NO3)3)?
Answers
4.56g excess reactant is left over when 17.0 g of potassium hydroxide (KOH) reacts with 20.0 g of iron (III) nitrate (Fe(NO₃)₃)
Reactants are raw materials that react with one another and form products.
Here given balanced reaction is
2KOH + Fe(NO₃)₂ → Fe(OH)₂ + 2KNO₃
Then we have to calculated the masses of KOH and Fe(NO₃)₂ from the balanced reaction
Molar mass of KOH = 39+16+1 = 56g/mol
Mass of KOH = 2×56 = 112g
And the molar mass of Fe(NO₃)₂ = 56+2[14+(16×3)]
= 56+2[14 + 48)]
= 56+2[62]
= 56+124
= 180g/mol
Then from the balanced equation
112g of KOH and 180g/mol of Fe(NO₃)₂
Then the 17 g of KOH = 17×180/112g
= 27.32 g of Fe(NO₃)₂
Then for 20.0 g of iron (III) nitrate
Therefore Xg of KOH = 112×20/180
Xg of KOH = 12.44g
Thus 12.44g of KOH reacted
Therefore we have determine the leftover mass of the excess reactant
Mass of KOH leftover = ?
Mass of KOH leftover = (Mass of KOH given) – (Mass of KOH that reacted)
Mass of KOH leftover = 17 - 12.44g
Mass of KOH leftover = 4.56g
Know more about potassium hydroxide
https://brainly.com/question/20592733
#SPJ1
Write and balance the following equation:
A solution of Sodium hydroxide reacts with a solution of iron (III)
nitrate to create a solid of iron (III) hydroxide in a solution of
sodium nitrate.
Answers
Balanced equation :
3NaOH + Fe(NO₃)₃⇒ Fe(OH)₃ + 3NaNO₃
Further explanationGiven
Word equation
Required
Balanced equation
Solution
Chemical equations can be expressed in terms of:
word equation skeleton equation balanced equationSodium hydroxide reacts with a solution of iron (III)
nitrate to create a solid of iron (III) hydroxide in a solution of
sodium nitrate.
We determine the chemical formula of each compound then we balance the chemical equation
Sodium hydroxide = NaOH
Iron (III)nitrate = Fe(NO₃)₃
Iron (III) hydroxide = Fe(OH)₃
Sodium nitrate = NaNO₃
Reaction
NaOH + Fe(NO₃)₃⇒ Fe(OH)₃ + NaNO₃
Give a coefficient(most complex compound = 1)
aNaOH + Fe(NO₃)₃⇒ bFe(OH)₃ + cNaNO₃
Fe, left = 1, right=b⇒b=1
N, left=3, right=c⇒c=3
O, left=a+9, right=3b+3c⇒a+9=3b+3c⇒a+9=3.1+3.3⇒a+9=12⇒a=3
The equation becomes :
3NaOH + Fe(NO₃)₃⇒ Fe(OH)₃ + 3NaNO₃
A beaker contains 1.0 M NaOH(aq) solution. To produce a NaOH(aq) solution of molar concentration 0.20 M, we transfer 2.0 ml (cm^3) out of this beaker into a 25 ml cylinder and add distilled water until the total volume reading becomes
A) 12ml
B)10ml
C)8ml
D)5ml
E)2ml
Answers
Answer: B) 10 ml
Explanation:
According to the dilution law,
\(M_1V_1=M_2V_2\)
where,
\(M_1\) = molarity of stock \(NaOH\) solution = 1.0 M
\(V_1\) = volume of stock \(NaOH\) solution = 2.0 ml
\(M_1\) = molarity of dilute \(NaOH\) solution = 0.20 M
\(V_1\) = volume of diluted \(NaOH\) solution = ?
Putting in the values we get:
\(1.0\times 2.0=0.20\times V_2\)
\(V_2=10ml\)
Therefore, final volume reading is 10 ml
HELP PLEASE 15 POINTS
The rate law for a reaction is found to be rate = k[X]4. By what factor does the rate increase if [X] is doubled?
Answers
1.-A mixture of aluminum metal and chlorine gas reacts to form aluminum chloride (AlCl3): 2Al(s) +
3Cl2(g) → 2AlCl3(s). How many moles of aluminum chloride will form when 5 moles of chlorine gas react
with excess aluminum metal?
Answers
The number of mole of aluminum chloride, AlCl₃ produced when 5 moles of chlorine gas, Cl₂ reacts is 3.33 moles
Balanced equation2Al(s) + 3Cl₂(g) → 2AlCl₃(s)
From the balanced equation above,
3 moles of Cl₂ reacted to produce 2 moles of AlCl₃.
How to determine the moles of AlCl₃ producedFrom the balanced equation above,
3 moles of Cl₂ reacted to produce 2 moles of AlCl₃.
Therefore,
5 moles of Cl₂ will react to = (5 × 2) / 3 = 3.33 moles of AlCl₃.
Thus, 3.33 moles of AlCl₃ were obtained from the reaction.
Learn more about stoichiometry:
https://brainly.com/question/14735801
The thioketal product of a certain reaction is given below. Draw the structure of: the organic reactant the protecting group reactant H r
Answers
Answer: The organic reactant is 1,3-propanedithiol. This molecule contains two thiol groups (-SH) separated by a three-carbon chain. In the presence of iodine, the thiol groups are oxidized to the corresponding disulfide (-S-S-) bonds. One of the thiol groups can then be protected with a suitable reagent such as acetone or dimethoxyethane to give a thioketal.
Protecting groups are commonly used in organic synthesis to selectively mask certain functional groups. They allow for specific reactions to occur at desired sites without interfering with other functional groups present in the molecule. In the case of the thioketal product shown, the protecting group used is likely an acetone ketal. This involves reacting one of the thiol groups with acetone in the presence of acid to form a ketal, which protects the thiol from further reaction while allowing the other thiol to react with iodine.
Know more about acetone ketal here:
https://brainly.com/question/14789253
#SPJ11
1). Which of the following best defines thermal equilibrium? A: Two substances' temperatures remain the same during transfer of heat energy B: The temperatures of substances never change during the transfer of heat C: All substances will eventually reach the same temperature D: Two substances reach equal temperatures during the transfer of heat energy
Answers
Answer:
A
Explanation:
at thermal equilibrium, temperature of 2 substances remain constant during heat transfer.
Select The on that most applys
Will mark brainliest

Answers
Answer:
A,C
Explanation:
Because the other question deal with convection but A,D show how the warm and cold water travel .
Obtain two new 1.5 mL microfuge tube. Close lids on them. Label one of them with your name, RNA sample and "250 ng/ul. You will prepare a dilution of your RNA sample so that it is exactly 250ng/uL concentration. Prepare 30 uL of this dilution using this formula: C1 V1=C2 V2
Answers
Using a micropipette, pipette 12 µL of the stock solution into the second labeled tube.5. Add 18 µL of nuclease-free water to the second labeled tube to bring the total volume up to 30 µL. This will give a final concentration of 250 ng/µL.
RNA or Ribonucleic acid is the genetic material that helps to transfer genetic information from DNA to protein synthesis. It is essential in decoding and regulation of genes. RNA isolation is the process of extracting RNA molecules from biological samples.The given formula C1V1
= C2V2 states that the concentration (C1) and volume (V1) of the initial solution is equal to the concentration (C2) and volume (V2) of the final solution. This means that the amount of solute (in this case, RNA) before and after dilution is the same. The formula can be used to calculate the volume of the stock solution required to prepare a dilution of a specific concentration.1. Label two new 1.5 mL microfuge tube with your name and RNA sample. Close the lids on them.2. Using a micropipette, pipette 30 µL of your RNA sample into one of the labeled tubes.3. Using the formula C1V1
= C2V2, we can calculate the volume of stock solution required to prepare the dilution.C1
= initial concentration
= unknown V1
= initial volume
= 30 µL (the volume we pipetted)C2
= final concentration
= 250 ng/µLV2
= final volume
= unknown (what we need to find)We can rearrange the formula to solve for V2:V2
= C1V1/C2V2
= (C1V1)/C2V2
= (100 ng/µL × 30 µL) ÷ 250 ng/µLV2
= 12 µL4.
Using a micropipette, pipette 12 µL of the stock solution into the second labeled tube.5. Add 18 µL of nuclease-free water to the second labeled tube to bring the total volume up to 30 µL. This will give a final concentration of 250 ng/µL.
To know more about micropipette visit:
https://brainly.com/question/30335063
#SPJ11
A dry, solid rock conducts electricity better than a long, meta wire.
True
False
Answers
Answer:
false
Explanation:
metal such as copper, silver, etc conducts heat and electricity. an object such as a rock, plastic, paper does NOT conduct heat.
Pre 3 & 4: Distillation and Fractional Distillation
Where should the thermometer bulb be to measure temperature accurately during
distillation?
Answers
The thermometer bulb should be placed at the same height as the distillate (the liquid being collected).
Thermometer bulb be to measure temperature accurately during distillation?The thermometer bulb should be placed at the same height as the distillate (the liquid being collected). This is because the temperature of the distillate is the most accurate representation of the temperature of the vapor being condensed. Placing the thermometer bulb at a different height than the distillate can result in inaccurate temperature readings, which can affect the efficiency and effectiveness of the distillation process.
In fractional distillation, there may be multiple fractions with different boiling points being collected, and the temperature may vary at different points in the fractionating column. In this case, multiple thermometers can be placed at different heights in the fractionating column to measure the temperature accurately at each point. This allows for better control over the separation of the different fractions based on their boiling points.
Learn more about Thermometer
brainly.com/question/21474245
#SPJ11
how many calories are required to boil 75 grams of water not in sig figs
Answers
Well, each ml of water requires one calorie to go up 1 degree Celsius, so this liter of water takes 1000 calories to go up 1 degree Celsius. (There are 1000 ml, each of which needs to have its temperature raised.)
The first element in each period has 1 valence electron and the last element in each period has _________ electronsA. 2 valence elementsB. 8 valence electronsC. 2 valence electronsD. 6 valence elements
Answers
8 valence electrons in B. That is accurate, indeed. Except for helium, which has just two valence electrons, the final element in each cycle, sometimes referred to as the noble gases, has eight valence electrons.
Valence is a phrase used to describe the emotional energy, either good or negative, attached to a stimulus or event. A stimulus or event is considered to have a high valence if it causes people to feel joy, happiness, or love. On the other hand, something is considered to have a poor valence when it causes others to feel sad, angry, or afraid. Valence can be arbitrary, which means that different people may respond emotionally differently to the same event or stimuli. The desire for good feelings and the avoidance of negative emotions are two universal valence patterns, according to studies. In disciplines like psychology, neurology, and marketing, valence is crucial since it can
Learn more about valence here:
https://brainly.com/question/12744547
#SPJ4
Bases and acids are _
A. Mixtures
B. Gases
C. opposites
D. The same
Answers
Answer:
opposite
Explanation:
mark me as a brainlist please
mole to mole rations

Answers
Based on the given equation: N₂+ 3 H₂ → 2 NH₃, the mole-to-mole ratios are:
a. N₂/H₂ - 1 : 3
b. N₂/NH₃ - 1 : 2
c. H₂/NH₃ - 3 : 2
KNO3
Based on the given equation: 8 H₂ + S₈ → 8 H₂S,the mole-to-mole ratios are:
a. H₂/H₂S - 1 : 1
b. H₂/S₈ - 8 : 1
3. Based on the given equation: 2 H₂ + O₂ → 2 H₂O
a. The H₂/H₂O mole-to-mole ratio is 1 : 1
b. Suppose you had 20 moles of H₂ on hand and plenty of O₂, the number of moles of H₂O you could make is 20 moles of H₂O.
C. The O₂ / H₂O mole to mole ratio is 1 : 2
d. Suppose you had 20 moles of O₂ and enough H₂, the number of moles of H₂O you could make is 40 moles.
What is the mole ratio of a reaction?The ratio of the mole quantities of any two compounds present in a balanced chemical reaction is known as the mole ratio. A comparison of the ratios of the molecules required to accomplish the reaction is given by the balancing chemical equation.
Learn more about mole ratio at: https://brainly.com/question/26023
#SPJ1
10. In a household radiator, 1000.g of steam at 100.°C condenses (changes from gas to
liquid). How much heat is released?
Answers
1000 g of water condenses at 100°C in a home radiator (changes from gas to liquid).
Equation
Q=m x Hvap
where m= mass.
= 1000. gx2260J/g
Q=2,260,000 J
What is Condensation?
Condensation, which is the opposite of vaporization, is the transformation of matter from its gaseous state into its liquid state. The water cycle is the most frequent use of the phrase. [1] Another way to describe it is as the transformation of water vapour into liquid water when it comes into touch with a solid, liquid, or cloud condensation nucleus in the atmosphere. Deposition is the term for the change that occurs when the gaseous phase directly transitions into the solid phase.
to know more about Condensation, visit to:-
https://brainly.com/question/5500097
#SPJ1
what is the volume of solute a and the volume of solvent b you need to mix in order to create a 1 l solution which is 0.5 m? density of a
Answers
To make a 1-liter solution with a concentration of 0.5 m, we need to determine the volume of solute and the volume of solvent necessary.
To begin, we need to understand that concentration is determined as mol/L or M. As a result, the number of moles of the solute must be 0.5 mol per liter.
A solution with a concentration of 0.5 mol/L means that 1 liter of the solution contains 0.5 moles of solute.
It's important to remember that the concentration of the solution is given by the following formula:
Molarity (M) = number of moles of solute/volume of solution in liters
M = 0.5 M = x / 1 liter
Number of moles of solute = 0.5 mol
Substitute the values in the above formula
0.5 M = x / 1 liter
x = 0.5 mol
We must know the density of the solvent in order to determine the volume of the solvent. In the absence of this, we cannot determine the volume of the solvent.
Learn more about solutions at brainly.com/question/30620786
#SPJ11
the oxidation number of a nitrogen atom in n₂o₃ is
Answers
A nitrogen atom in N2O3 has an oxidation number of +3.
The unknown nitrogen oxidation number can be given a variable (x) to ascertain its oxidation number. Since oxygen has an oxidation number of - 2 and there are three oxygen particles in N₂O₃, the complete negative charge from oxygen is (- 2) × 3 = - 6.
The total charge of a compound is equal to the sum of its oxidation numbers. Since the compound in question is neutral, the sum of the oxidation numbers must be zero in this instance.
2(N) + 3(O) = 0
2x + (-6) = 0
2x = 6
x = 3
To learn more about oxidation numbers:
https://brainly.com/question/4222605
How many mols of H3PO4 are produced when 284 g P4O10 reacts completely to form H3PO4? P4O10 + 6H2O --> 4H3PO4
Answers
Answer:
4 moles of H3PO4
Explanation:
Molar mass of P4O10 = 284 g/mol
Number of moles of P4O10 reacted = 284g/284 g/mol = 1 mole of P4O10
From the reeaction equation;
1 mole of P4O10 yields 4 moles of H3PO4
Hence, 284 g of P4O10 yields 4 moles of H3PO4
answer this question fast please!

Answers
Answer:
A is a physical change, no atoms are being lost its still in it original form
B is a chemical change, it is losing atoms and changing into a new substance
Explanation:
In your own words, explain why it is necessary to include only one chain-terminating/ synthesis-terminating nucleotide in each well of the electrophoresis instrument.
Answers
Answer: DNA sequencing methods employ chain termination nucleotides. Furthermore, if two chain-terminating nucleotides are employed in the same well, determining which strand is ended by which dideoxynucleotide will be impossible.
(its my own words, but i did search it to get this answer, hope it helps!)
Learn more about chain-terminating here:
https://brainly.com/question/3476061
#SPJ2
In a container with volume of 25.0 L, there are 40 g of CH4 gas. If the number of gas is reduced to 15.0 L, what is the new amount inmole?
Answers
Answer
1.50 mol
Explanation
Given:
Initial volume, V₁ = 25.0 L
Mass of CH4 gas in 25.0 L container = 40 g
Final volume, V₂ = 15.0 L
From the Periodic Table; molar mass of CH4 = 16.04 g/mol
What to find:
The new amount in mole.
Step-by-step solution:
According to Avogadro’s law: For a confined gas, the volume (V) and number of moles (n) are directly proportional if the pressure and temperature both remain constant. That is:
\(\frac{V_1}{n_1}=\frac{V_2}{n_2}\)n₁ = Mass/Molar mass = (40.0g/16.04 g/mol) = 2.493765586 mol
n₂ is the new amount in mole and can be calculated as follows:
\(\begin{gathered} \frac{25.0\text{ L}}{2.493765586\text{ mol}}=\frac{15.0\text{ L}}{n_2} \\ \text{Cross multiply} \\ n_2\times25.0\text{ L }=15.0\text{ L }\times2.493765586\text{ mol} \\ \text{Divide both sides by 25.0 L} \\ \frac{n_2\times25.0\text{ L}}{25.0\text{ L}}=\frac{15.0\text{ L }\times2.493765586\text{ mol}}{25.0\text{ L}} \\ n_2=1.496259352\text{ mol} \\ To\text{ 3 significant digits} \\ n_2=1.50\text{ mol} \end{gathered}\)The new amount in moles is 1.50 moles