Chemists and physicists often have to manipulate and control a variable that they want to study. Scientists who need to control a variable must perform which type of scientific investigation?
A. experimental
C. comparative
B. free exploration
D. observational
Answers
Related Questions
Find the volume of a regular shaped block with a measurement of 12 cm long, 8 cm wide and 14 cm high.
Answers
Answer:
1344 \(cm^{3}\)
Explanation:
Volume = length x width x height
Here:
Length = 12 cm
width = 8 cm
height = 14 cm
Substituting the values in the formula:
Volume = 12 x 8 x 14
= 1344 \(cm^{3}\)
Read the chemical equation.
Fe2O3 + CO -> Fe + CO2
If 2 moles of Fe2O3 react with 9 moles of CO, how many moles of each product are formed?
Answers
When 2 mole of Fe2O3 and 9 molecules of CO react, 6 moles of the each product are created.
Describe a mole.The term "mole" refers to a common scientific measurement unit for atoms, molecules, and other very small particles.
The first one is supposed to produce a balanced chemical reaction in accordance with the situation supplied.
FeO3 + 3 CO 2 Fe + 3CO2 is the formula.
Because 2 moles of Fe2O3 reacted with 3 x2=6 moles of Co to produce Fe and CO2, CO was in excess and Fe2O3 was the limiting reagent.
Utilize the mole ratio to determine the moles of the each product.
That is the Fe2O3 mole ratio: Because Fe is a 1:2 element, the mole of Fe is 2x2=4 moles.
Because the mole ratio of Fe2CO3 to CO2 is 1:3, the moles of Co2 are 2 x 3 = 6 moles.
Therefore, 6 moles of the each product will be created when 2 moles or Fe2O3 react to 9 moles of CO.
To know more about chemical equation visit:
https://brainly.com/question/30087623
#SPJ1
If you add 169 calories of heat energy to 63.5 g of Fe (s), how much will the temperature of the iron (Fe) change?
The specific heat of iron is 0.11 cal/g*C
Answers
Answer:
38 cal. Explanation: The key to this problem lies in the value of the specific heat of iron. ciron=0.108 cal g−1∘C−1.
Explanation:
_______ bonding is similar to ionic bonding, except there are no high-electronegativity atoms present to accept any electrons that the present atoms are willing to donate.
Answers
Covalent bonding is similar to ionic bonding, except there are no high-electronegativity atoms present to accept any electrons that the present atoms are willing to donate.
Covalent bonding is a type of bonding that occurs when two atoms share electrons. It takes place between atoms of similar electronegativity, meaning they have the same attraction for electrons, and occurs when the atoms’ orbitals overlap in a stable electronic configuration. This overlap allows electrons to be shared between the two atoms in equal proportions and binds them together, forming a covalent bond.
This is in contrast to ionic bonding, where one atom has a higher electronegativity and is able to accept electrons from the other with a lower electronegativity. Thus, covalent and ionic bonds tend to form between atoms of different electronegativities.
In covalent bonding, there are no polar molecules or ions, meaning there is no charge difference between atoms. It’s also much stronger than ionic bonding, because the shared electrons are held more tightly by the atoms due to the attractive forces between them.
know more about electrons here
https://brainly.com/question/12001116#
#SPJ11
What is the mass of 4.80 mol of barium hydride, BaH2?
A. 4.80 g
B. 29.0 g
C. 139 g
D. 669 g
Answers
Answer:
mole=wt/M.wt
M.wBaH2→139.34g/mol
4.80=wt/139.34
wt=4.80×139.34
wt=668.8
wt≈669✓
According to mole concept, the mass of 4.80 mole of barium hydride is 669 g.
What is a mole?Mole is defined as the unit of amount of substance . It is the quantity measure of amount of substance of how many elementary particles are present in a given substance.
It is defined as exactly 6.022×10²³ elementary entities. The elementary entity can be a molecule, atom ion depending on the type of substance. Amount of elementary entities in a mole is called as Avogadro's number.
It is widely used in chemistry as a suitable way for expressing amounts of reactants and products.For the practical purposes, mass of one mole of compound in grams is approximately equal to mass of one molecule of compound measured in Daltons. Molar mass has units of gram per mole . In case of molecules, where molar mass in grams present in one mole of atoms is its atomic mass.
Number of moles is calculated as, mass/molar mass ,therefore on substitution mass=number of moles×molar mass
mass= 4.80×139.34=668.83 ≈669 g.
Therefore, the mass of 4.80 mole of barium hydride is 669 g.
Learn more about moles,here:
https://brainly.com/question/26416088
#SPJ6
Identify the hybridization of the N atoms in N2H4
Answers
All of the nitrogen in the N2H4 molecule hybridizes to Sp3.
How does hybridization work?The combining of two atomic orbitals to create a whole new class of hybridized orbitals is the notion of hybridization in chemistry. Hybrid orbitals with completely distinct energies are often the outcome of this mixing. In hybridization, the same-energy level atomic orbitals are crucial. However, as long as they have an equivalent amount of energy, both fully and partially filled orbitals can participate in this process.
All of the nitrogen in the N2H4 molecule hybridizes to Sp3. N2H4 has a dipole moment of 1.85 D and is polar in nature. The nitrogen atoms in N2H4 have no formal charge. The N2H4 molecule has a trigonal molecular geometry (shape).
To know more about hybridization, go to link
https://brainly.com/question/22765530
#SPJ9
Garlic and onions are often sautéed in butter beforebeing used in recipes. Why do chefs do this?
A) Their flavors and/or odors are fat-soluble
B) They need to be cooked to rid them of bacteria
C) They take longer to be cooked than otheringredients
D) Their proteinsneed to be denaturedbefore consumption
Answers
Chefs sauté garlic and onions in butter before using them in recipes primarily because their flavors and/or odors are fat-soluble. This process helps to enhance the taste and aroma of the dish.
Garlic and onions contain volatile compounds that contribute to their distinct flavours and aromas. These compounds are soluble in fats and oils rather than in water. By sautéing them in butter, which is a fat, these compounds are released and dissolved, allowing their flavours to infuse into the dish. The fat-soluble nature of these compounds enables them to disperse more evenly and intensify the overall taste profile of the recipe. Additionally, sautéing garlic and onions in butter also helps to soften their texture and mellow their flavours. The gentle cooking process allows the natural sugars in onions to caramelize, resulting in a sweeter taste. It also helps to reduce the pungency and sharpness of garlic, making it more palatable.
To learn more about sautéed click here: brainly.com/question/11715001
#SPJ11
Any observations collected from Parts A and B were obtained by testing solutions with a single cation present. Would the presence of two cations in one solution (like that of the unknown) affect the cation identification in Part C?
A. No, at room temperature the blue pigment cation will react with the reagent for a particular test allowing you to identify the first cation. When you heat the solution up, the white pigment cation reacts with that reagent and can be identified.
B. Yes, the two cations in the unknown can react with the reagent added for a particular test. The observation of the reaction for one cation can mask the other cation in solution making it difficult to identify either cation.
C. Yes, the cations can react with each other to form a precipitate. Therefore, the cations are no longer available to react with the reagent added for a particular test making it impossible to identify the cations.
D. No, it does not matter how many cations are in solution. After centrifugation, the less dense white pigment cation will be at the top and the more dense blue pigment cation will be at the bottom. This makes cation identification very straight forward.
Answers
The presence of two cations in the unknown solution can affect cation identification in Part C. This is because the cations can react with each other to form a precipitate, which will make it impossible to identify either cation.
The cations can also react with the reagent added for a particular test and the observation of the reaction for one cation can mask the other cation in solution, making it difficult to identify either cation.
In addition, the two cations can have different densities, which can make cation identification difficult. Thus, the presence of two cations in a solution can affect cation identification. It is important to take this into account when performing the cation identification experiment.
Know more about precipitate here
https://brainly.com/question/18109776#
#SPJ11
What might happen if the mouse was removed from this
food web? Please be specific and mention ALL organisms
affected.
I’ll give u brainliest hurry
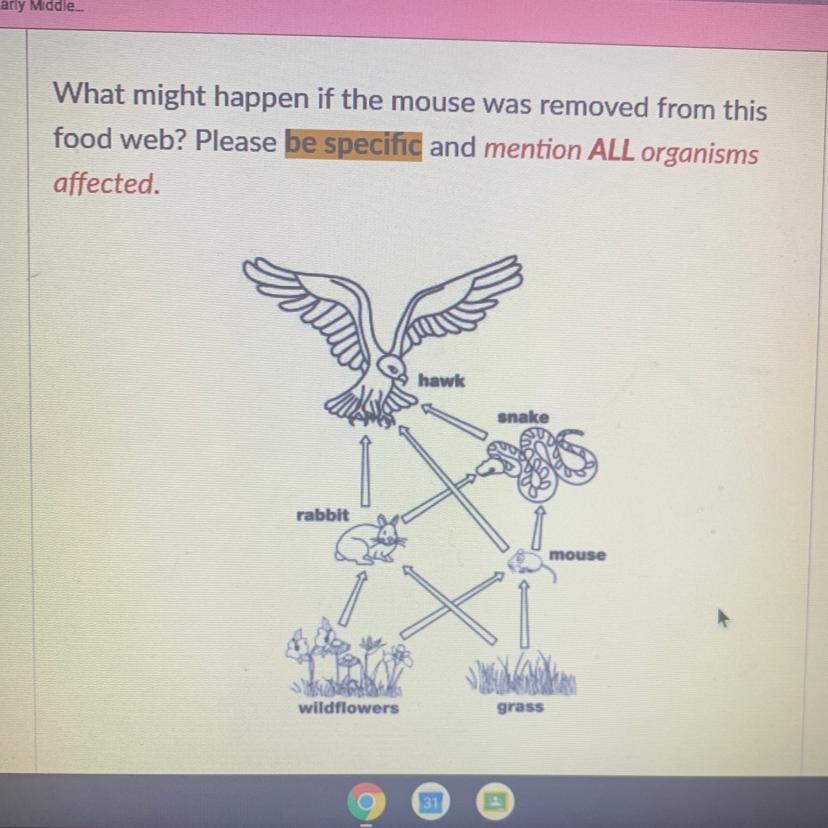
Answers
Answer:
They would most likely starve and die UNLESS they could move to another habitat or tend to eating other sources of food. All the other animals in the food web would die too, because their food supplies would have gone. The populations of the consumers would fall as the population of the producer fell.
When a organism is removed, the organism who eats or hunt them will decrease some because they lost one of the food source even though they still have other food sources. This new organism will brake the balance of the ecosystem so their food sources will decrease by having too many predators that hunt and eat them.
In food chain's if one source from it is ever removed it would mess up the entire chain causing issue
If you have 144 moles of As , you need moles of NaOH use it all up

Answers
ANSWER
The number of moles of NaOH is 432 moles
EXPLANATION
Given information
\(2\text{ As + 6NaOH }\rightarrow\text{ 2Na}_3AS_{O3}+\text{ 3H}_2\)The number of moles of As = 144 moles
Let x represents the number of moles of NaOH
From the above reaction, you will see that 2 moles of As react with 6 moles of NaOH
The next step is to find the number of moles of NaOH using a stoichiometry ratio
\(\begin{gathered} 2\text{ moles of As }\rightarrow\text{ 6 moles of NaOH} \\ 144\text{ moles of As }\rightarrow\text{ x moles of NaOH} \\ \text{ Cross multiply} \\ 2\text{ }\times\text{ x = 6 }\times144 \\ 2x\text{ = 864} \\ \text{ Divide both sides by 2} \\ \frac{2x}{2}\text{ = }\frac{864}{2} \\ x\text{ = 432 moles} \end{gathered}\)Hence, the number of moles of NaOH is 432 moles
write the electron configuration for fe3 ?express your answer in condensed form, in order of increasing orbital energy. for example, [he]2s22p2 would be entered as [he]2s^22p^2.
Answers
The electron configuration for Fe^3+ in condensed form, in order of increasing orbital energy, is: [Ar]3d^5.
Here is a detailed explanation of how to write this electron configuration:
* The symbol [Ar] represents the noble gas configuration of argon, which has 18 electrons. This is a shorthand notation that allows us to avoid writing out the full electron configuration of argon.
* The next part of the electron configuration, 3d5, represents the five electrons in the 3d orbital of Fe3. The 3d orbital is a subshell of the 3rd energy level, and it can hold up to 10 electrons.
* The order of the orbitals in the electron configuration is important, and it follows the Aufbau principle, which states that electrons fill orbitals in order of increasing orbital energy.
Therefore, the electron configuration for Fe3 is [Ar]3d5.
This electron configuration reflects the fact that Fe3 has lost three electrons from its 4s orbital, leaving five electrons in the 3d orbital. The 3d orbital is the most stable orbital for these electrons, so they will occupy this orbital before they occupy any of the higher energy orbitals.
Learn more about electron configuration https://brainly.com/question/26084288
#SPJ11
What is the definition of percent composition in your own words?
Answers
Answer:
percentage by mass of each element in a compound.
Explanation:
calculate the weight of kclo3 that would be required to produce 29.5 l of oxygen measured at 127oc and 760 torr.
Answers
The weight of KClO₃ that would required to produce 29.5 l of oxygen measured at 127 C is 73.53 grams .
Molar mass :The mass in grams of one mole of a substance is known as molar mass. The abbreviation for molar mass is g/mol, which stands for grams per mole. A value that is proportional to the mass of the isotope carbon-12 is referred to as the isotopic atomic mass of a single elemental isotope. The ratio of the mass to the amount of substance in any sample of a chemical compound is known as its molar mass. A substance's molar mass is a bulk property rather than a molecular one.
Evaluating :Temperature = 127 °C = 400 K
Pressure = 760 torr = 1 atm
n = PV / RT
= 1*29.5 / ( 0.082 × 400)
= 0.9
2KClO₃ = 2 KCl + 3O₂
Moles of KClO₃ = 2/3 moles of O₂
= 2/3 × 0.9
= 0.6
Mass of KClO₃ = mole × molar mass
= 0.6 × 122.55
= 73.53 grams
Learn more about molar mass :
brainly.com/question/21334167
#SPJ1
When Mg bonds with S, which of the following is true?a. Mg and S are in a "sea of electrons."b.Mg and S share two electrons.c. Mg gains two electrons, while S loses two electrons.d. Mg loses two electrons, while S gains two electrons.
Answers
Answer: Magnesium loses two electrons whilst sulfur gains tw
Observe the following images. Which of them shows a chemical change happening?
I is rusting car, II is water boiling, III is cooking eggs and IV is melting ice cream
A. I
B. I and III
C. II and IIII
D. I, II, and III
Answers
Answer: B
Explanation:
I. Rusting is a chemical reaction which is oxidation of iron.
II. Boiling water is a physical change. There is no chemical change between H2O molecules. It only shifts to the gas state from the liquid state.
III. When egg is cooked proteins are denaturated so it changes interactions between molecules.
IV. Melting is also just a phase change like boiling.
How to identify polar molecules
Answers
Answer:
Polar molecules occur when there is an electronegativity difference between the bonded atoms. Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out
Explanation:
a gas mixture called heliox, 6.11% o2 and 93.89% he by mass, is used in scuba tanks for descents more than 65 m below the surface. calculate the mole fractions of he and o2 in this mixture.
Answers
The mole fractions of He and O₂ in this mixture are 0.992 and 0.008 respectively.
The mole fraction can be calculated by way of dividing the number of moles of 1 factor of an answer via the full variety of moles of all the additives of an answer. it is mentioned that the sum of the mole fraction of all the components inside the solution has to be the same as one.
Mole Fraction describes the range of molecules contained within one aspect divided through the whole range of molecules in a given combination. it's miles quite beneficial whilst two reactive-natured components are mixed collectively.
O₂ = 6.11g / 32.0g = 0.191 mol O2
He = 93.89g / 4 = 23.5 mol He
Mole fraction = Mole fraction A = Moles A / Total moles present in a mixture
Mole fraction O₂ = 0.191 mol O₂ / Total moles (0.191 mol O2 + 23.5 mol He)
0.191 O₂ / 23.7 total moles (O2 + He)
0.008 Mole fraction O₂
For He
Mole fraction He = 23.5 mol He / 23.7 total moles
Mole fraction He = 0.992
Learn more about mole fractions here:-https://brainly.com/question/14783710
#SPJ4
Which of the following are spectator ions in the equation below? Select all that apply
Pb(C₂H302)2(ag) + 2 LiCl(ag) 2 LIC₂H302(ag) + PbCl2(s)
Pb2+(aq)
Li+(aq)
Cl-(aq)
C2H3O-2(aq)
I hope i entered the answer options right
Answers
write the steps used for making tea by using the words: solution, solvent,solute,dissolve,soluble, insoluble, filtrate and residue.
answer
Answers
Answer:
Water is solvent and milk, tea powder and sugar is solute.
Explanation:
First take a specific mount of water in teapot which is a solvent and heat it for several minutes. After boiling of water, add 1 to 2 table spoon tea powder in water which is a solute and allow it for boiling. After that add milk and sugar according to your preference and tea is made which is a solution. The milk and sugar dissolve in the water but the tea powder did not dissolve so it can be filtrate with the help of filtration tool and all the residue trap at that tool while the tea is clean from all undissolved particles.
Determine the charge of each ion.
al oxygen ion with 10 electrons
(b) aluminum ion with 10 electrons
(c) titanium ion with 18 electrons
(d) iodine ion with 54 electrons
Answers
Answer:
(a) ==> O²-
(b) ==> Al³+
(c) ==> Ti²+
(d) ==> I-
What causes elements to react to each other
Answers
Answer:
One atom of each element is made up of protons, neutrons, and electrons. The number of electrons determines how an element reacts. The number of protons gives the element its identity. ... They react well with nonmetals because they can easily give up electrons to form ions.
What is a tipping point? What will happen if the climate reaches a tipping point?
Answers
Answer:
The Intergovernmental Panel on Climate Change (IPCC) defines tipping points as “critical thresholds in a system that, when exceeded, can lead to a significant change in the state of the system, often with an understanding that the change is irreversible.”
Explanation:
The decomposition of N2O4 is studied at 20oC and 80oC. Which statement explains why the rate at 80oC is greater than at 20oC?
a.The activation energy is higher at 80oC.
b.The activation energy is lower at 80oC.
c.The concentration of a gas increases with increasing temperature.
d.The number of molecules with enough energy to react is greater at 80oC.
Answers
The correct statement is d. The number of molecules with enough energy to react is greater at 80oC.
This is due to the fact that at higher temperatures, the molecules of N2O4 possess more kinetic energy which increases the frequency of collisions and the proportion of molecules that exceed the activation energy required for the reaction to occur.
As a result, the rate of the reaction increases at higher temperatures. The activation energy of a reaction is the minimum energy required for a chemical reaction to occur, but it remains constant regardless of the temperature. Therefore, options a and b are incorrect.
Option c is also incorrect as the concentration of the gas does not increase with temperature, but its pressure does.
To know more about temperatures. please visit.....
brainly.com/question/31166060
#SPJ11
You are about to take a short trip. However, you will not be traveling by car or bus; instead you will be traveling up in a hot air balloon. You know that hot air balloons, like the ones pictured, are able to rise when the air contained in them is heated. Why does the balloon rise? What is happening at the molecular level to make the balloon rise?
Answers
Answer:
Hot air rises. Heated air molecules “spread out” or expand and bounce around, and the space becomes less dense than the surrounding space. Increasing the air temperature inside the balloon envelope makes it less dense than the air, thus making it “lighter than air”.
Explanation:
I'm new to brainly but I hope this helped!
Balloon rise because Warm air ascends. Heat causes the molecules of the air to "spread out," or expand, and bounce off one another, making the area less dense than the surroundings.
What are molecules ?
A molecule is the smallest unit of a substance that keeps its content and properties. It is made up of two or more atoms that are joined together by chemical bonds.
Chemistry is built on molecules. The element symbol and a subscript indicating the number of atoms are used to identify molecules.
When Balloon rise because Warm air ascends. Heat causes the molecules of the air to "spread out," or expand, and bounce off one another, making the area less dense than the surroundings.
The air inside the balloon's envelope becomes less dense as the temperature rises, making it "lighter than air."
To learn more about molecules, refer to the below link:
https://brainly.com/question/14130817
# SPJ2
At a certain temperature, the equilibrium constant K for the following reaction is 819.: Use this information to complete the following table. Suppose a 38. L reaction vessel is filled with 0.51 mole of CO_2 and 0.51 mole of H_2. What can you say about the composition of the mixture in the vessel at equilibrium? What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. There will be very little CO and H_2O. There will be very little CO_2 and H_2. Neither of the above is true.
Answers
At equilibrium, the composition of the reaction vessel with 0.51 moles of CO₂ and 0.51 moles of H₂ is given by the equilibrium constant K, which is 819.
The reaction given is:
H₂(g) + CO₂(g) ⇌ CO(g) + H₂O(g)
So, we know the formula for Kp and Kc in terms of partial pressure and concentration are as follows:
Kc = (CO(g) × H₂O (g)) / (CO₂(g) × H₂(g))
Kp = (PCO × PH₂O) / (PCO₂ × PH₂)
At equilibrium, the equilibrium constant expression can be written as:
Kc = [CO][H₂O] / [H₂][CO₂] = x²/ [(0.51-x)(0.51-x)]
Substituting the given value of Kc into the above equation and solving for x gives:
819 = x²/[(0.51-x)(0.51-x)]
x = 0.302
From the results, we can say that "There will be very little CO₂ and H₂" at equilibrium.
The equilibrium constant for the given reaction is 819.
Learn more about equilibrium constants here:
https://brainly.com/question/3159758
#SPJ11
Balance this reaction:
C6H10Cl4+Cl2=
Answers
Answer:
C6H10 + 2Cl2 → C6H10Cl4
Explanation:
which of the following best describes a functional group? multiple choice question. large molecules comprised of a glycerol and three fatty acid chains large molecules composed of two or more repeating smaller units special combinations of atoms that attach to hydrocarbon chains and rings to form organic nutrients molecules that are composed of hydrogen and carbon in a long chain or ring-like structure
Answers
The answer is (b). Specialized atom combinations known as functional groups bind to hydrocarbon chains and rings to create organic nutrition.
Any of the countless atom combinations that compose chemical compounds and undertake distinctive reactions on their own are categorized as functional groups. It frequently affects the remaining molecules in each molecule's reactivity. The idea of functional groups can be used as a foundation for categorizing numerous substances based on how they behave. Common functional group examples include the hydroxyl found in alcohols and phenols, the carboxyl found in carboxylic acids, and the carbonyl found in aldehydes, ketones, and quinones. It can be simply defined as an atom or group of atoms inside a molecule that share chemical characteristics when they appear in multiple compounds, despite the fact that other components of the molecule are very different.
Learn more about functional groups here
https://brainly.com/question/30666298
#SPJ4
The Complete question is
which of the following best describes a functional group? multiple choice question. large molecules comprised of a glycerol and three fatty acid chains large molecules composed of two or more repeating smaller units special combinations of atoms that attach to hydrocarbon chains and rings to form organic nutrients molecules that are composed of hydrogen and carbon in a long chain or ring-like structure
(a) large molecules comprised of a glycerol and three fatty acid chains
(b) special combinations of atoms that attach to hydrocarbon chains and rings to form organic nutrients
(c) molecules that are composed of hydrogen and carbon in a long chain or ring-like structure
(d) large molecules composed of two or more repeating smaller units
In general, foods high in saturated fatty acids are found in which food groups?
Select one:
a. Fruits and vegetables
b. Milk and meat
c. Vegetable oils
d. Starches and breads
Answers
In general, foods high in saturated fatty acids are found in milk and meat food groups (Option B).
What are saturated fats?Saturated fats are those that are solid at room temperature and have no double bonds between their carbon atoms. Saturated fats are commonly found in foods of animal origin, such as fatty beef, pork, and poultry, as well as in butter, cheese, and other dairy products.
Lipids that are solid at ambient temperature are known as saturated fatty acids. They are present in meat and dairy products, including cheese, milk, and butter, as well as some plants, including coconut oil, cocoa butter, and palm oil. Foods high in saturated fats are usually of animal origin and are linked to an increased risk of heart disease.
Thus, the correct option is B.
Learn more about Saturated fats: https://brainly.com/question/29816448
#SPJ11
Please answer it in 1 hour Write explanation if it needed I’ll give you upvote immediately Don’t use excel to solve this question i In a bond amortization schedule, what does the book value mean?Describe in words. (ii) Consider a n-period coupon bond where the redemption amount, C may not be the same as the face amount, F. Using j and g to represent the yield rate per period and modified coupon rate per period respectively, show that,for k = 01,2,n, the book value at time k,B is B=C+Cg-jan-kj and the amortized amount at time k is ii Let K = Cu. The Makeham formula to compute the price of a bond is given by A verbal interpretation for K would be that K is the present value of the redemption value C.Provide a verbal interpretation for(C-K)
Answers
Answer:
(i) In a bond amortization schedule, the book value represents the remaining amount of the bond principal that hasn't been paid off at a given point in time. When a bond is first issued, its book value equals its face value. As payments are made over the life of the bond, a portion of these payments reduces the book value. By the end of the bond's life, its book value will be zero, as the entire principal will have been paid off.
(ii) The formula for the book value B at time k, where k is the number of periods elapsed, is B = C + Cg - jan-kj.
Here:
- C is the redemption amount,
- g is the modified coupon rate per period,
- j is the yield rate per period, and
- a_n-kj is the present value of an annuity immediate with n - k periods at the yield rate j.
This formula states that the book value at any time k is the redemption amount plus the present value of the future coupon payments (Cg), minus the present value of the annuity that represents the repayments of the bond (jan-kj).
The amortized amount at time k is the change in the book value from time k-1 to time k, plus the coupon payment at time k. It represents the portion of the bond's principal (and interest) that has been repaid up to time k.
(iii) If K is defined as the present value of the redemption value C, according to the Makeham formula, (C-K) would represent the difference between the redemption value of the bond and its present value. This difference is the amount of interest that will accumulate over the life of the bond. In other words, (C-K) can be interpreted as the total interest that the bondholder will earn from holding the bond until redemption, assuming that all coupon payments are reinvested at the yield rate j.
Explanation:
What is the difference between intermolecular forces of attraction and covalent bonds and how do I know if its strong or weak
Answers
Answer:
An intermolecular force is the force of attraction or repulsion which act between neighboring particles (atoms, molecules, or ions). Whereas the covalent bonds are the interatomic linkage that results from the sharing of an electron pair between two atoms.
Intermolecular forces hold molecules together in a liquid or solid. Intermolecular forces are generally much weaker than covalent bonds.