Answers
Answer: 28.02 g
Explanation:
The M stands for molarity. It is moles of solute/liters of solution. We can use the molarity to convert liters to mL, then make a proportion to find the grams.
\(\frac{2.75 mol}{L} *\frac{1L}{1000mL} =\frac{2.75 mol}{1000mL}\)
Now that we have molarity in moles and mL, we can use the 107mL to get moles.
\(\frac{2.75moles}{1000mL} *107mL=0.29425mol\)
We would multiply moles by molar mass to get grams. The molar mass of magnesium chloride is 95.211 g/mol.
\(0.29425mol*\frac{95.211g}{mol} =28.02g\)
Related Questions
How much water has to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M?
Answers
Approximately 166.67 mL of water needs to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M.
To find the amount of water that needs to be evaporatedThe relationship between the initial and final concentrations and volumes must be taken into account.
Given: Initial concentration \((C^1) = 1 M Initial volume (V^1) = 250 mL\)
\((C^2) = 3 M final concentration\)
We can use the equation:
\(C^1 * V^1 = C^2 * V^2\)
Where:
\(V^2\)is the final volume of the solution
Rearranging the equation to solve for V2:
\(V^2 = (C^1 * V^1) / C^2\)
Substituting the given values:
\(V^2 = (1 M * 250 mL) / 3 M\)
\(V^2 = 250 mL / 3\)
\(V^2\) ≈ \(83.33 mL\)
To find the amount of water that needs to be evaporated, we subtract the final volume from the initial volume:
Amount of water to be evaporated = \(V^1 - V^2\)
Amount of water to be evaporated = 250 mL - 83.33 mL
Amount of water to be evaporated ≈ 166.67 mL
Therefore, approximately 166.67 mL of water needs to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M.
Learn more about Initial concentration here: brainly.com/question/30720317
#SPJ1
The theory of evolution states
Answers
Answer:
the theory of evolution states that all living things which exist today, and many more that are now extinct, evolved from simple life forms which first developed 3 billion years ago.
Explanation:
2. Determine the possible traits of the calves of : Da red (RR) bull is mated with a red (RR) cow 1 a red * (RR) bullis mated with a white (WW) Cow 2 Da roan * (RW) is mated with a red(RR)Cow 3 3. Illustrate your answers using a Punnett square. 4. Write your answers on the paper.
Answers
2nd square - all 4 roan babies
3rd square - 2 red, 2 roan babies

What is one way that minerals crystallize from materials dissolved in water?
Answers
Answer:
From solutions that evaporate
From hot water solutions when the solution cools
Explanation:
The substances that can form minerals can be dissolved in the water to form a solution. Solution can be described as as a mixture in which a solute is dissolved in a solvent. When a particular dissolved solute evaporates from the solution, crystals of minerals might form. The mineral halite was formed when seas evaporated over a period of time.
The minerals can also be formed when a hot water solution gets cooled.
which gas is fossil fuel
Answers
Answer:
methane
Explanation: methane is obtained from the decaying of flora and fauna mostlyunder damp
What is the main difference between the Eubacteria and Archaebacteria kingdoms and the protista, fungi, plantae and Animalia kingdoms
Answers
The main difference between the Eubacteria and Archaebacteria kingdoms and the protista, fungi, plantae, and animal kingdoms is that Eubacteria and Archaebacteria are prokaryotes, while protista, fungi, plantae, and animal kingdoms are eukaryotes.
What is the Six Kingdoms Classification?In the six kingdoms of classification, there are Eubacteria, Archaebacteria, Protista, Fungi, Plantae, and Animalia. The Eubacteria is a prokaryote, and its cell membrane is made up of peptidoglycan, teichoic acid, etc. Archaea are also prokaryotes, but their cell membrane composition differs from that of bacteria, so they are classified separately.
The eukaryotes are further classified into protista, fungi, plantae, and animalia depending upon their feeding patterns, complexity, etc. Protists may be unicellular or multicellular. Plants are autotrophs, fungi and animals are heterotrophs.
Eubacteria and Archaebacteria are examples of prokaryotes, whereas protists, fungi, plantae, and animals are examples of eukaryotes.
Learn more about the Six Kingdoms Classification, here
https://brainly.com/question/3748453
#SPJ1
Identify the type of reaction and predict the product: Calcium + water -->
Answers
Answer:
Exothermic Reaction
Product = Calcium hydroxide + hydrogen
Explanation:
exactly 149.6J will raise the temperature of 10.0g of a metal from 25.0C. what is the specific heat capacity of the metal
Answers
Exactly 149.6J will raise the temperature of 10.0g of a metal from 25.0C. The specific heat capacity of the metal is 5.984 J/g°C.
What is specific heat capacity?The heat capacity of a sample of a substance divided by the mass of the sample yields the specific heat capacity (symbol c), also known as massic heat capacity. Informally, it is the quantity of heat that must be added to one unit of a substance's mass in order to raise its temperature by one unit. The specific heat capacity unit in the SI is the joule per kelvin per kilogram, or Jkg⁻¹K⁻¹. For instance, the specific heat capacity of water is 4184 J kg⁻¹K⁻¹, or the amount of energy needed to raise 1 kilogram of water by 1 K.
The specific heat capacity of the metal can be calculated using the equation Q = m × c ×ΔT.
Q = 149.6J
m = 10.0g
ΔT = (final Temperature - initial Temperature) = (25°C - 0°C) = 25°C
Plugging these values into the equation, we get:
149.6J = 10.0g × c ×25°C
Solving for c, we get:
c = \(\frac{149.6J}{(10.0g *25C)}\)
c = 5.984 J/g°C
Therefore, the specific heat capacity of the metal is 5.984 J/g°C.
To know more about specific heat capacity, visit:
https://brainly.com/question/29766819
#SPJ1
1. HF (aq) = H+ (aq) + F- (aq)
K 6.8 x 10-4
-
II. H₂C₂O4 (aq) = 2H+ (aq) + C₂0²-
K = 3.8 x 10-6
What is the K value for the
reaction below?
2HF (aq) + C₂02 (aq) =
2F (aq) + H₂C₂O4 (aq)
[?]
K = [?] x 10¹

Answers
Remember:
when you double moles: square the equilibrium constant
when you half the moles: Square root the equilibrium constant
when you reverse a reaction: take the reciprocal of the equilibrium constant

Use standard enthalpies of formation to calculate ΔH∘rxn for the following reaction. 3NO2(g)+H2O(l)→2HNO3(aq)+NO(g)
Answers
The standard enthalpy change (∆H°rxn) for the given reaction is -38.0 kJ/mol. This negative value indicates that the reaction is exothermic, meaning that it releases heat to the surroundings.
The standard enthalpies of formation (∆Hf°) for the reactants and products involved in the reaction are: ∆Hf°[NO2(g)] = +33.2 kJ/mol. ∆Hf°[H2O(l)] = -285.8 kJ/mol. ∆Hf°[HNO3(aq)] = -207.2 kJ/mol. ∆Hf°[NO(g)] = +90.4 kJ/mol. We can use these values to calculate the standard enthalpy change (∆H°) for the reaction using the following equation:
∆H°rxn = Σn∆Hf°(products) - Σm∆Hf°(reactants) where n and m are the stoichiometric coefficients of the products and reactants, respectively. Substituting the values, we get:∆H°rxn = [2 × ∆Hf°(HNO3(aq))] + [∆Hf°(NO(g))] - [3 × ∆Hf°(NO2(g))] - [1 × ∆Hf°(H2O(l))]. ∆H°rxn = [2 × (-207.2 kJ/mol)] + [90.4 kJ/mol] - [3 × (+33.2 kJ/mol)] - [1 × (-285.8 kJ/mol)]. ∆H°rxn = -414.4 kJ/mol + 90.4 kJ/mol - 99.6 kJ/mol + 285.8 kJ/mol. ∆H°rxn = -38.0 kJ/mol
Therefore, the standard enthalpy change (∆H°rxn) for the given reaction is -38.0 kJ/mol. This negative value indicates that the reaction is exothermic, meaning that it releases heat to the surroundings.
For more such question on enthalpy visit:
https://brainly.com/question/16985375
#SPJ11
Check all the formulas below that represent binary compounds.
NH4CL
CaS
P₂05
CaSO4
N₂O
Cl₂
Answers
Binary compound is a substance that is made up of precisely two separate components and cannot be further simplified chemically. Binary compounds include, for instance, H₂O, H₂S, and NH₃.
1. NH₄Cl - It is not a binary compound because it contains more than two elements that is Nitrogen, hydrogen and chlorine.
2. CaS - It is a binary compound because it contains two elements Calcium and sulfur.
3. P₂O₅ - It is a binary compound because it contains two elements Phosphorus and Oxygen.
3. CaSO₄ - It is not a binary compound because it contains more than two elements that is calcium, sulfur and oxygen.
4. N₂O - It is a binary compound because it contains two elements that is Nitrogen and Oxygen.
5. Cl₂- It is not a binary compound because it does not contain two different elements rather it is a diatomic molecule.
Diatomic molecules- Two atoms are chemically linked together to form diatomic molecules. A homonuclear diatomic molecule is created when two identical atoms combine, such as in the oxygen molecule (O₂). A heteronuclear diatomic molecule is created when two unidentical atoms combine, such as in the CO₂ .
To learn more about compounds refer- https://brainly.com/question/26487468
#SPJ9
Not a timed or graded assignment. Please use CER format. (Claim, evidence, reasoning) Quick answer = amazing review thank you so much In advance :)

Answers
Calculations -
1. We are given
• moles of Al2O3 = 9.45 moles
,• Molar mass of AL2O3 =(2* 26,981539) + (3* 15.999)
= 101,96 g/mol
2. We can calculate the mass of Al2O3 :
\(\begin{gathered} \text{Moles = }\frac{mass\text{ Al2O3 }}{\text{Molar mass Al2O3 }} \\ \text{ }\therefore\text{ Mass = Moles }\cdot\text{ Molar Mass } \\ \text{ = 9.45 moles }\cdot\frac{101.96moles}{\text{gram}} \\ \text{ = 963.52 grams } \end{gathered}\)• This means that mass of AL2O3 = 963.52 grams
,• As a result, Jacob miscalculated the amount of mass of Al2O3 because he divided moles by mol. mass instead of multiplying moles by molar mass .
What forms of energy are produced when
fossil fuels burn?
Answers
When fossil fuels burn, several forms of energy are produced, including:
Heat energy: The primary form of energy released during fossil fuel combustion is heat. Fossil fuels contain chemical energy stored for millions of years, and when they burn, this energy is released in the form of heat. The heat energy can be harnessed for various purposes, such as heating buildings or generating steam to drive turbines.
Light energy: Burning fossil fuels can also produce light energy in the form of flames or glowing embers. This light energy is a byproduct of combustion.
Mechanical energy: Heat generated by burning fossil fuels can be converted into mechanical energy. This is typically achieved by using heat to produce steam, which drives a turbine connected to a generator. The rotating turbine converts the heat energy into mechanical energy, which is further transformed into electrical energy.
Electrical energy: Through the process described above, burning fossil fuels can ultimately generate electrical energy. The mechanical energy produced by the turbine is converted into electrical energy by the generator. Electrical energy can power various devices, appliances, industries, and infrastructure.
It's critical to note that while burning fossil fuels can produce useful forms of energy, it also results in the release of carbon dioxide and other greenhouse gases. This contributes to climate change and environmental concerns. As a result, there is a global shift towards cleaner and renewable energy sources to mitigate these negative impacts.
Calculate the pH of a buffer solution that contains 0.25 M benzoic acid (C 6H 5CO 2H) and 0.15M sodium benzoate (C
Answers
Answer:
\(pH=3.97\)
Explanation:
Hello,
In this case, for the calculation of the pH of the given buffer we need to use the Henderson-Hasselbach equation:
\(pH=pKa+log(\frac{[base]}{[acid]} )\)
Whereas the pKa for benzoic acid is 4.19, the concentration of the base is 0.15 M (sodium benzoate) and the concentration of the acid is 0.25 M (benzoic acid), therefore, the pH turns out:
\(pH=4.19+log(\frac{0.15M}{0.25M} )\\\\pH=3.97\)
Regards.
I learned that.......
Mixtures are the combination of
substances
that can be homogeneous or heterogeneous, Homogeneous mixtures
mixtures, while heterogeneous mixtures are
mixtures.
are
Answers
A mixture could be homogeneous or heterogeneous.
A mixture refers to a combination of two or more substances that are not chemically combined together. They may be solids, liquids or gases.
A mixture may be composed of substances in the same phase or different phases. If substances are combined in the same phase to form a mixture then the mixture is homogeneous. If substances in different phases combine to give a mixture then the mixture is heterogeneous.
Examples of homogeneous mixtures are salt and water solutions while examples of heterogeneous mixtures are sand and water as well as oil and water.
Learn more: https://brainly.com/question/12160179
I need help on balancing the equations and on what type of reaction it is.

Answers
Given the balanced chemical reaction expressed as:
\(2Au_2O_3\rightarrow4Au+3O_2\)A Redox reaction is a reaction that involves the transfer of electrons and changes in the oxidation state between the elements.
The given chemical equation is therefore an oxidation-reduction reaction since it involves a change in the oxidation state of the elements.
Determine the moles of Au₂O₃
\(\begin{gathered} \text{Mole = }\frac{Mass}{Molar\text{ mass}} \\ \text{Mole of Au}_2O_3=\frac{10g}{441.93g\text{/mol}} \\ \text{Mole of Au}_2O_3=0.02263\text{moles} \end{gathered}\)According to stochiometry, you can see that 2 moles of Gold(III)oxide produce 4 moles of Gold. Hence the moles of Gold produced will be:
\(\begin{gathered} \text{moles of Gold=}\frac{0.02263\times4}{2} \\ \text{moles of Gold=}0.02263\times2 \\ \text{moles of Gold=}0.0453\text{moles} \end{gathered}\)Determine the mass of Gold.
\(\begin{gathered} \text{Mass of Gold=moles}\times molar\text{ mass} \\ \text{Mass of Gold=}0.0453\times196.97 \\ \text{Mass of Gold}=8.91\text{grams} \end{gathered}\)Next is determining the mole of Oxygen
According to stochiometry, you can see that 2 moles of Gold(III)oxide produce 3 moles of Oxygen. Hence the moles of Oxygen produced will be:
\(\begin{gathered} \text{moles of Oxygen=}\frac{0.02263\times3}{2} \\ \text{moles of Oxygen}=0.033945\text{moles} \end{gathered}\)Determine the mass of oxygen produced
\(\begin{gathered} \text{Mass of O}_2=moles\times\text{Molar mass} \\ \text{Mass of O}_2=0.033945\times16 \\ \text{Mass of O}_2=0.543\text{grams} \end{gathered}\)Hence the mass of oxygen produced is 0.543 grams
Determine the empirical formula of a compound containing 48.38 grams of carbon, 8.12 grams of hydrogen, and 53.5 grams of oxygen
Answers
As with most stoichiometry problems, it is necessary to work in moles. The ratio of the moles of each element will provide the ratio of the atoms of each element.
Get the mass of each element by assuming a certain overall mass for the sample (100 g is a good mass to assume when working with percentages).
Remeber that percentages are a ratio multiplied by 100. You must convert percentages back to their decimal value before working with them.
(.4838) (100 g) = 48.38 g C
(.0812 ) (100 g) = 8.12 g H
(.5350) (100 g) = 53.38 g O
Convert the mass of each element to moles of each element using the atomic masses.
(48.38 g C) (1 mol/ 12.10 g C) = 4.028 mol C
(8.12 g H) (1 mol/ 1.008 g H) = 8.056 mol H
(53.38 g O) (1 mol/ 16.00 g O) = 3.336 mol O
Find the ratio or the moles of each element by dividing the number of moles of each by the smallest number of moles.
Use the mole ratio to write the empirical formula.
Which of the following transitions (in a hydrogen atom) represent emission of the shortest wavelength photon?Group of answer choices:n = 3 to n = 1n = 2 to n = 1n = 4 to n = 1n = 4 to n = 3n = 4 to n = 2
Answers
In this question , we will have to calculate the wavelength for each of the given answer choices, the shortest wavelength is the answer .
1. n = 3 to n = 1
we will use rydberg formula for wavelength : let represent wavelength.
1/ = RH * z^2 ( 1/n1^2 - 1/n2^2) ......{n1 = 1 , n2 = 3}
1/ = RH * z^2( 1/1-1/9)
1/ = 1.09x10^7*1 (8/9)
∴ =1.03x10^-7
2. n = 2 to n = 1
1/ = RH * z^2 ( 1/1^2 - 1/2^2)
=1.09x10^7*1 ( 3/4)
1/ =8175000
∴ =1.22x10^-7
3. n = 4 to n = 1
1/ = -10218750
∴ = -9.7x10^-8
4 . n = 4 to n = 3
1/ = -529861.1
∴ = -1.88x10 ^-6
5. n = 4 to n = 2
1/ = -4768750
∴ =-4.89x10^-7
• Finally, we can see that option C is the most negative value, ,∴, = -9.7x10^-8
When the suns radiant energy for the Earth oceans, it causes water to change state by that rating which form of energy does water vapor have
Answers
Water vapor has latent heat energy, which is absorbed or released during the process of changing states from liquid to gas or gas to liquid.When the sun's radiant energy hits the Earth's oceans, it causes the water molecules to absorb this energy and become more energized.
This leads to the water molecules breaking apart and transforming into water vapor, which is a gaseous state of water. Water vapor has a specific form of energy known as latent heat. This is the energy required to change the physical state of water from a liquid to a gas or from a gas to a liquid. The process of converting water into water vapor requires energy, and this energy is stored in the water vapor in the form of latent heat.
The amount of latent heat absorbed or released by water vapor is dependent on the temperature and pressure conditions. When the water vapor condenses back into liquid form, this latent heat is released into the atmosphere. This process plays a critical role in weather and climate patterns as it drives the movement of heat and moisture throughout the Earth's atmosphere.
In summary, water vapor has latent heat energy, which is absorbed or released during the process of changing states from liquid to gas or gas to liquid. This energy plays a vital role in the Earth's weather patterns and is a critical component of the Earth's energy balance.
For more such questions on heat energy
https://brainly.com/question/934320
#SPJ11
How does maximum boiling azeotropic mixture is separated using fractional distillation?
Answers
Answer:
By heating the mixture to maximum boiling point and then the solution is distilled at a constant temperature without having a change in composition.
Explanation:
An azeotropic mixture is also called a constant boiling mixture and it is a mixture of two or more liquids whose proportions cannot be altered by simple distillation due to the fact that when an azeotropic mixture is boiled, the vapor has the same proportions of constituents as the unboiled mixture.
Now, maximum boiling azeotropic mixture are the solutions with negative deviations that have an intermediate composition for which the vapor pressure of the solution is minimum and as a result, the boiling point is maximum. At that point, the solution will distill at a constant temperature without having a change in composition.
which of the following is the unit of surface tension?
Answers
Answer:
it should be N/m or newton per meter.
What are two processes that must occur to form soil?
Question 1 options:
weathering breaks rocks into minerals and plants die and decay
erosion and weathering
Plants produce loam and plants produce humus
erosion transports mineral particles and plants die and decay
Answers
Erosion and weathering are two processes that must occur to form soil.
What is soil formation?Soil formation is the process by which soil is created over time through the physical, chemical, and biological interactions between rocks, minerals, organic matter, water, air, and living organisms.
Soil formation is a slow and complex process that can take centuries or even millennia, and it can be influenced by a variety of factors, including climate, topography, parent material, time, and human activities.
What is erosion and weathering?Weathering refers to the physical and chemical processes that break down rocks and minerals at or near the Earth's surface.
Erosion, on the other hand, refers to the movement and transport of weathered materials, such as soil, rock fragments, and sediment, by water, wind, or glaciers. This can result in the reshaping of landscapes, the creation of new landforms, and the deposition of sediments in new locations.
Learn about Weathering here https://brainly.com/question/829782
#SPJ1
MARKING AS BRAINLIEST! LAST ATTEMPT.(2 questions in one photo )
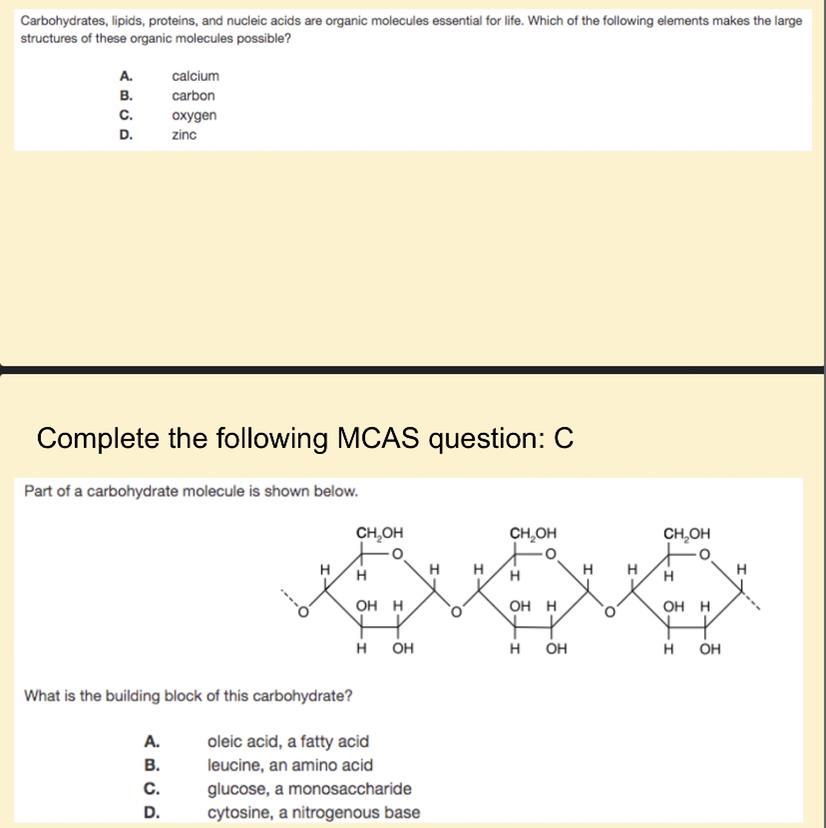
Answers
Answer:
b. carbon; c. glucose
Explanation:
"Nonetheless, all organisms are built from the same six essential elemental ingredients: carbon, hydrogen, "nitrogen, oxygen, phosphorus and sulfur (CHNOPS)." -lifescience
Cathodic protection of iron involves using another more reactive metal as a sacrificial anode. Classify each of the following metals by whether they would or would not act as a sacrificial anode to iron under standard conditions.
a. Ag
b. Mg
c. Cu
d. Pb
e. Sn
f. Zn
g. Au
Answers
Answer:
a. Ag ---> cannot serve as a sacrificial anode for iron because it is lower than iron in the reactivity series. Hence, it is less reactive than iron.
b. Mg ---> can serve as a sacrificial anode for iron because it is higher than iron in the reactivity series. Hence, it is more reactive than iron.
c. Cu ---> cannot serve as a sacrificial anode for iron because it is lower than iron in the reactivity series. Hence, it is less reactive than iron.
d. Pb ---> cannot serve as a sacrificial anode for iron because it is lower than iron in the reactivity series. Hence, it is less reactive than iron.
e. Sn ---> cannot serve as a sacrificial anode for iron because it is lower than iron in the reactivity series. Hence, it is less reactive than iron.
f. Zn ---> can serve as a sacrificial anode for iron because it is higher than iron in the reactivity series. Hence, it is more reactive than iron.
g. Au ---> cannot serve as a sacrificial anode for iron because it is lower than iron in the reactivity series. Hence, it is less reactive than iron.
Explanation:
Cathodic protection of iron involves using another more reactive metal as a sacrificial anode. The reactivity series of metals arranges metals based on decreasing order of reactivity. The more reactive metals are found higher up in the series while the least reactive metals are found at the lower ends of the series. Thus, metals above iron in the reactivity series can serve as sacrificial anodes by protecting against corrosion, while those lower than iron cannot.
Based on the reactivity series, the following metals can be classified as either a sacrificial anode for iron or not:
a. Ag ---> cannot serve as a sacrificial anode for iron because it is lower than iron in the reactivity series. Hence, it is less reactive than iron.
b. Mg ---> can serve as a sacrificial anode for iron because it is higher than iron in the reactivity series. Hence, it is more reactive than iron.
c. Cu ---> cannot serve as a sacrificial anode for iron because it is lower than iron in the reactivity series. Hence, it is less reactive than iron.
d. Pb ---> cannot serve as a sacrificial anode for iron because it is lower than iron in the reactivity series. Hence, it is less reactive than iron.
e. Sn ---> cannot serve as a sacrificial anode for iron because it is lower than iron in the reactivity series. Hence, it is less reactive than iron.
f. Zn ---> can serve as a sacrificial anode for iron because it is higher than iron in the reactivity series. Hence, it is more reactive than iron.
g. Au ---> cannot serve as a sacrificial anode for iron because it is lower than iron in the reactivity series. Hence, it is less reactive than iron.
A+ 2B
An elementary liquid phase reaction needs to be carried out in a CSTR reactor with a
volume 5 m3 and conversion desired is 70%.the molar feed is 30 % A and 70% B at a
pressure 202 kpa and 333к
1. Construct a complete stoichiometric table in terms of concentrations
2. What is the rate of reaction of A
3. Calculate k & E and then specify the type of reaction energy
Additional information:
Total feed: 10 mole/s.
Gas constant: 8.314 kJ/mol.oK
Frequeney factor: 0.00717 m'/mols
Answers
The stoichiometric table and the rate law for the given elementary liquid phase reaction have been constructed. The rate constant and activation energy have been calculated, and the type of reaction energy has been specified as endothermic.
Stoichiometric table in terms of concentrations:
The stoichiometric table for the given reaction can be constructed as follows:
A + 2B → products
A B products
Feed 0.3*Cf 0.7*Cf 0
Exit (0.3-0.3X)*C (0.7-0.7X)*C 0
Change -0.3XC -0.7XC 0
Where:
Cf = Total feed concentration
C = Concentration inside reactor
X = Conversion of A
Rate of reaction of A:
The rate of the reaction can be expressed as:
rA = -1/2 * dCA/dt = k*C^2
where, CA is the concentration of A and k is the rate constant.
Since the reaction is elementary, the rate law is proportional to the concentrations of the reactants raised to their stoichiometric coefficients.
The rate of disappearance of A = rate of appearance of B
rB = -dCB/dt = 2*rA
Therefore, the rate of reaction of A can be expressed as:
rA = (0.7Cf - 0.7C)/V = k*C^2
Substituting values, we get:
rA = (0.710 - 0.70.7X)/5 = k(0.3 - 0.3*X)^2
Calculation of k and E:
The rate constant k can be calculated using the Arrhenius equation:
k = A * exp(-Ea/RT)
where A is the frequency factor, Ea is the activation energy, R is the gas constant and T is the temperature in Kelvin.
Assuming the activation energy is 50 kJ/mol, we can calculate the rate constant at the given temperature of 333 K:
k = 0.00717 * exp(-50000/(8.314*333)) = 0.0001504
The reaction energy can be determined by calculating the activation energy using the rate constant at two different temperatures. Assuming the rate constant at 323 K is 0.000098, we can solve for Ea:
ln(k2/k1) = Ea/R * (1/T1 - 1/T2)
ln(0.000098/0.0001504) = Ea/8.314 * (1/323 - 1/333)
Ea = 43775 J/mol
The positive value of the activation energy indicates that the reaction is endothermic.
for more questions on reaction
https://brainly.com/question/18095210
#SPJ11
The meaning of the word symptom:
Answers
The word "symptom" refers to a specific manifestation or indication of a condition, disease, or disorder that is experienced or observed by an individual.
Symptoms are subjective or objective changes in the body's normal functioning that may be recognized as abnormal, uncomfortable, or problematic. Symptoms can manifest in various ways depending on the nature of the underlying condition. They can be physical, such as pain, rash, cough, fever, or fatigue, indicating an illness or injury affecting the body. Symptoms can also be psychological, such as anxiety, depression, or confusion, reflecting disturbances in mental health.
Symptoms serve as important clues for medical professionals to identify and diagnose diseases or disorders. They provide valuable information about the nature, severity, and progression of an illness, helping healthcare providers formulate appropriate treatment plans. Additionally, symptoms may also be important for individuals to self-assess their own health status and seek appropriate medical attention.
It is essential to note that symptoms alone may not provide a definitive diagnosis, as they can overlap across different conditions. Further evaluation, including medical tests and examinations, is often necessary to confirm a diagnosis and determine the appropriate course of action.
for more such questions on symptom
https://brainly.com/question/21078887
#SPJ8
87.653 g of lead(II) oxide > moles of lead(II) oxide
Answers
The number of mole present in 87.653 g of lead(II) oxide is 0.393 mole
Description of moleThe mole of a substance is related to it's mass and molar mass according to the following equation:
Mole = mass / molar mass
With the above formula, we can determine the mole of lead(II) oxide. Details below:
How to determine the mole present in 87.653 g of lead(II) oxideMass of lead(II) oxide = 87.653 g Molar mass of lead(II) oxide = 223.2 g/molMole of lead(II) oxide =?Mole = mass / molar mass
Mole of lead(II) oxide = 87.653 / 223.2
Mole of lead(II) oxide = 0.393 mole
Thus, 87.653 g of lead(II) oxide contains 0.393 mole
Learn more about mole:
https://brainly.com/question/13314627
#SPJ1
4. What is an example of a chemical change that happens inside your body?
a. Food being broken down by enzymes in your stomach b. Food being broken into small pieces by your teeth
c. Food being moved from your esophagus into your stomach
d. Water being absorbed inside your large intestine
Answers
Answer: a
Explanation: enzymes are a type of chemical in your body that helps break down food, thus it’s a chemical change in your body.
Food is broken down by the enzymes in the stomach is an example of chemical change happening inside the body. So the correct option is A.
What is digestion?
The process of breakdown down food into its constituent nutrients is defined as the process of digestion. The process of digestion is of two types -
Mechanical digestion: This includes the breakdown of food by mastication performed by teeth. During this process, the food is also mixed with the saliva and a bolus is formed. Chemical digestion: This includes the breakdown of food by the use of enzymes like the saliva present in salivary amylase and the gastric enzymes and intestinal enzymes from the stomach and intestines respectively.Thus, digestion begins in the mouth and both mechanical and chemical digestion takes place in the mouth. In the small intestine, the food is broken down into the smallest constituent nutrients and absorbed into the body.
Therefore the correct option is A.
Read more about digestion, here
https://brainly.com/question/1283194
#SPJ6
hot metal plate at 150°C haA hot metal plate at 150°C has been placed in air at room temperature. Which event would most likely take place over the next few minutes?
Molecules in both the metal and the surrounding air will start moving at lower speeds. Molecules in both the metal and the surrounding air will start moving at higher speeds. The air molecules that are surrounding the metal will slow down, and the molecules in the metal will speed up. The air molecules that are surrounding the metal will speed up, and the molecules in the metal will slow down.s been placed in air at room temperature. Which event would most likely take place over the next few minutes? Molecules in both the metal and the surrounding air will start moving at lower speeds. Molecules in both the metal and the surrounding air will start moving at higher speeds. The air molecules that are surrounding the metal will slow down, and the molecules in the metal will speed up. The air molecules that are surrounding the metal will speed up, and the molecules in the metal will slow down.
Answers
The air molecules that are surrounding the metal will speed up, and the molecules in the metal will slow down.
Heat transfer mechanismWhen a hot metal plate is placed in air at room temperature, heat transfer occurs between the metal and the surrounding air. The metal plate, being at a higher temperature, transfers heat to the surrounding air through conduction and convection. As a result, the air molecules that are in direct contact with the hot metal will gain energy and start moving at higher speeds.
On the other hand, the molecules in the metal plate will lose energy to the cooler air and, consequently, slow down. This is due to the transfer of thermal energy from the metal to the air.
Therefore, the most likely event over the next few minutes is that the air molecules surrounding the metal will speed up, and the molecules in the metal will slow down.
More on heat transfer can be found here: https://brainly.com/question/13433948
#SPJ1
When a hot metal plate at 150°C is placed in the air at room temperature, the heat transfers from the hot object to the cooler one - the warmer metal plate's molecules will slow down as they lose heat and the cooler air molecules surrounding it will speed up as they gain heat.
Explanation:If a hot metal plate at 150°C is placed in the air at room temperature, the event that would most likely happen over the next few minutes is that the air molecules that are surrounding the metal will speed up, and the molecules in the metal will slow down.
Explanation: Heat typically transfers from hotter objects to cooler objects according to the laws of thermodynamics. So, when a heated metal plate is placed in a cooler environment, the heat energy from the metal plate will transfer to the cooler air molecules. As a result, the air molecules will begin to move faster and the metal molecules will begin to slow down as the metal plate cools. This process continues until the metal plate and the air have reached a state of thermal equilibrium, which is when they have the same temperature.
Learn more about Heat Transfer here:https://brainly.com/question/34419089
#SPJ2
Classify H2O as cation, anion or neither
Answers
Answer:
H²O is neither a cation or anion
Explanation:
Hydrogen can form a cation (H+) or an anion (H-‐). Hydrogen in water (H20), ammonia (NH3), methane (CH4) and millions of other compounds is neither a cation nor an anion.